Glutamate Dehydrogenase, a Complex Enzyme at a Crucial Metabolic Branch Point
- PMID: 29079932
- PMCID: PMC5924581
- DOI: 10.1007/s11064-017-2428-0
Glutamate Dehydrogenase, a Complex Enzyme at a Crucial Metabolic Branch Point
Abstract
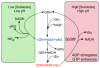
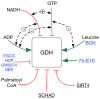
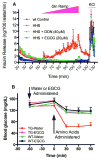
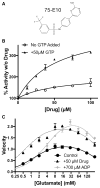
In-vitro, glutamate dehydrogenase (GDH) catalyzes the reversible oxidative deamination of glutamate to α-ketoglutarate (α-KG). GDH is found in all organisms, but in animals is allosterically regulated by a wide array of metabolites. For many years, it was not at all clear why animals required such complex control. Further, in both standard textbooks and some research publications, there has been some controversy as to the directionality of the reaction. Here we review recent work demonstrating that GDH operates mainly in the catabolic direction in-vivo and that the finely tuned network of allosteric regulators allows GDH to meet the varied needs in a wide range of tissues in animals. Finally, we review the progress in using pharmacological agents to activate or inhibit GDH that could impact a wide range of pathologies from insulin disorders to tumor growth.
Keywords: Allostery; Glutamate dehydrogenase; Insulin.
Figures
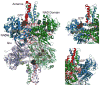

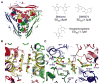

References
-
- Hudson RC, Daniel RM. L-Glutamate dehydrogenases: distribution, properties and mechanism. Comp Biochem Physiol. 1993;106B:767–792. - PubMed
-
- Frieden C. Glutamate dehydrogenase VI: survey of purine nucleotides and other effects on the enzyme from various sources. J Biol Chem. 1965;240:2028–2037. - PubMed
-
- Frieden C. Glutamic dehydrogenase I. The effect of coenzyme on the sedimentation velocity and kinetic mechanism. J Biol Chem. 1959;234:809–814. - PubMed
-
- Tomkins GM, Yielding KL, Curran JF. The influence of diethylstilbestrol and adenosine diphosphate on pyridine nucleotide coenzyme binding by glutamic dehydrogenase. J Biol Chem. 1962;237:1704–1708. - PubMed
-
- Bailey JS, Bell ET, Bell JE. Regulation of bovine glutamate dehydrogenase. J Biol Chem. 1982;257:5579–5583. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

