Safety, pharmacokinetics, metabolism and radiation dosimetry of 18F-tetrafluoroborate (18F-TFB) in healthy human subjects
- PMID: 29080017
- PMCID: PMC5660009
- DOI: 10.1186/s13550-017-0337-5
Safety, pharmacokinetics, metabolism and radiation dosimetry of 18F-tetrafluoroborate (18F-TFB) in healthy human subjects
Abstract
Background: 18F-Tetrafluoroborate (18F-TFB) is a promising iodide analog for PET imaging of thyroid cancer and sodium/iodide symporter (NIS) reporter activity in viral therapy applications. The aim of this study was to evaluate the safety, pharmacokinetics, biodistribution, and radiation dosimetry of high-specific activity 18F-TFB in healthy human subjects.
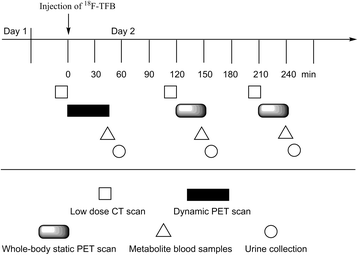
Methods: 18F-TFB was synthesized with specific activity of 3.2 ± 1.3 GBq/μmol (at the end of synthesis). Dynamic and whole-body static PET/CT scans over 4 h were performed after intravenous administration of 18F-TFB (333-407 MBq) in four female and four male healthy volunteers (35 ± 11 years old). Samples of venous blood and urine were collected over the imaging period and analyzed by ion-chromatography HPLC to determine tracer stability. Vital signs and clinical laboratory safety assays were measured to evaluate safety.
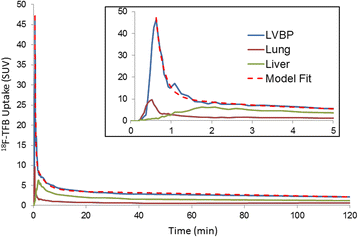
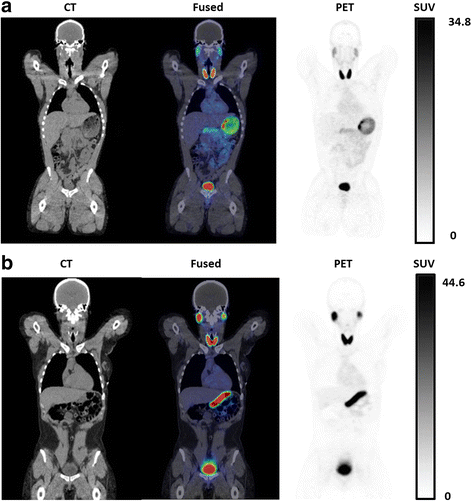
Results: 18F-TFB administration was well tolerated with no significant findings on vital signs and no clinically meaningful changes in clinical laboratory assays. Left-ventricular blood pool time-activity curves showed a multi-phasic blood clearance of 18F-radioactivity with the two rapid clearance phases over the first 20 min, followed by a slower clearance phase. HPLC analysis showed insignificant 18F-labeled metabolites in the blood and urine over the length of the study (4 h). High uptakes were seen in the thyroid, stomach, salivary glands, and bladder. Urinary clearance of 18F-TFB was prominent. Metabolic stability was evidenced by low accumulation of 18F-radioactivity in the bone. Effective doses were 0.036 mSv/MBq in males and 0.064 mSv/MBq in females (p = 0.08, not significant).
Conclusions: This initial study in healthy human subjects showed 18F-TFB was safe and distributed in the human body similar to other iodide analogs. These data support further translational studies with 18F-TFB as NIS gene reporter and imaging biomarker for thyroid cancer and other disease processes that import iodide.
Keywords: 18F-fluorine; Biodistribution; Dosimetry; PET imaging; Sodium/iodide symporter; Tetrafluoroborate.
Conflict of interest statement
Ethics approval and consent to participate
All participants provided informed consent. Approval of the study was obtained from the Mayo Clinic Institutional Review Board (no. 15-02494). All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent for publication
Consent to publish was obtained from all participants.
Competing interests
Dr. Stephen Russell owns stock and is founder of Vyriad. Dr. Russell owns stock and is co-founder of Imanis Life Sciences. Dr. Kah Whye Peng owns stock and is co-founder of Imanis Life Sciences. The other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Chung JK. Sodium iodide symporter: its role in nuclear medicine. J Nucl Med. 2002;43:1188–1200. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

