Chronic Recurrent Multifocal Osteomyelitis (CRMO): Presentation, Pathogenesis, and Treatment
- PMID: 29080202
- PMCID: PMC5705736
- DOI: 10.1007/s11914-017-0405-9
Chronic Recurrent Multifocal Osteomyelitis (CRMO): Presentation, Pathogenesis, and Treatment
Abstract
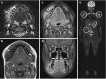
Purpose of review: Chronic non-bacterial osteomyelitis (CNO) with its most severe form chronic recurrent multifocal osteomyelitis (CRMO) is an autoinflammatory bone disorder. We summarize the clinical presentation, diagnostic approaches, most recent advances in understanding the pathophysiology, and available treatment options and outcomes in CNO/CRMO.
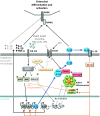
Recent findings: Though the exact molecular pathophysiology of CNO/CRMO remains somewhat elusive, it appears likely that variable defects in the TLR4/MAPK/inflammasome signaling cascade result in an imbalance between pro- and anti-inflammatory cytokine expressions in monocytes from CNO/CRMO patients. In this context, we present previously unpublished data on cytokine and chemokine expression in monocytes and tissues. CNO/CRMO is an autoinflammatory bone disorder resulting from imbalanced cytokine expression from innate immune cells. Though the exact molecular pathophysiology remains unclear, variable molecular defects appear to result in inflammasome activation and pro-inflammatory cytokine expression in monocytes from CNO/CRMO patients. Recent advances suggest signaling pathways and single molecules as biomarkers for CNO/CRMO as well as future treatment targets.
Keywords: Biomarkers; Bone; CNO; CRMO; Chronic non-bacterial osteomyelitis; Chronic recurrent multifocal osteomyelitis; Cytokine; Inflammation; Treatment.
Conflict of interest statement
Conflict of Interest
Jessica Pablik, Sigrun Hofmann, Herman Girschick, Polly Ferguson, Franz Kapplusch, Henner Morbach, and Christian Hedrich declare no conflict of interest.
Human and Animal Rights and Informed Consent
Studies in human tissue samples and primary human cells were approved by the local ethical committees by TU Dresden and University of Wuerzburg. Individuals or their legal guardians gave written informed consent.
Figures
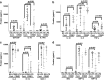


References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

