Effect of Autologous Cord Blood Infusion on Motor Function and Brain Connectivity in Young Children with Cerebral Palsy: A Randomized, Placebo-Controlled Trial
- PMID: 29080265
- PMCID: PMC5702515
- DOI: 10.1002/sctm.17-0102
Effect of Autologous Cord Blood Infusion on Motor Function and Brain Connectivity in Young Children with Cerebral Palsy: A Randomized, Placebo-Controlled Trial
Abstract
Cerebral palsy (CP) is a condition affecting young children that causes lifelong disabilities. Umbilical cord blood cells improve motor function in experimental systems via paracrine signaling. After demonstrating safety, we conducted a phase II trial of autologous cord blood (ACB) infusion in children with CP to test whether ACB could improve function (ClinicalTrials.gov, NCT01147653; IND 14360). In this double-blind, placebo-controlled, crossover study of a single intravenous infusion of 1-5 × 107 total nucleated cells per kilogram of ACB, children ages 1 to 6 years with CP were randomly assigned to receive ACB or placebo at baseline, followed by the alternate infusion 1 year later. Motor function and magnetic resonance imaging brain connectivity studies were performed at baseline, 1, and 2 years post-treatment. The primary endpoint was change in motor function 1 year after baseline infusion. Additional analyses were performed at 2 years. Sixty-three children (median age 2.1 years) were randomized to treatment (n = 32) or placebo (n = 31) at baseline. Although there was no difference in mean change in Gross Motor Function Measure-66 (GMFM-66) scores at 1 year between placebo and treated groups, a dosing effect was identified. In an analysis 1 year post-ACB treatment, those who received doses ≥2 × 107 /kg demonstrated significantly greater increases in GMFM-66 scores above those predicted by age and severity, as well as in Peabody Developmental Motor Scales-2 Gross Motor Quotient scores and normalized brain connectivity. Results of this study suggest that appropriately dosed ACB infusion improves brain connectivity and gross motor function in young children with CP. Stem Cells Translational Medicine 2017;6:2071-2078.
Keywords: Autologous stem cell transplantation; Cellular therapy; Clinical Trials; Cord blood; Human cord blood; Nervous system; Umbilical cord blood.
© 2017 The Authors Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Figures
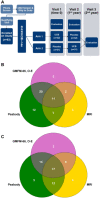
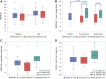
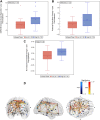
References
-
- Arneson CL, Durkin MS, Benedict RE et al. Prevalence of cerebral palsy: Autism and Developmental Disabilities Monitoring Network, three sites, United States, 2004. Disabil Health J 2009;2:45–48. - PubMed
-
- Meier C, Middelanis J, Wasielewski B et al. Spastic paresis after perinatal brain damage in rats is reduced by human cord blood mononuclear cells. Pediatr Res 2006;59:244–249. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

