Factor XIII cotreatment with hemostatic agents in hemophilia A increases fibrin α-chain crosslinking
- PMID: 29080382
- PMCID: PMC5802369
- DOI: 10.1111/jth.13887
Factor XIII cotreatment with hemostatic agents in hemophilia A increases fibrin α-chain crosslinking
Abstract
Essentials Factor XIII (FXIII)-mediated fibrin crosslinking is delayed in hemophilia. We determined effects of FXIII cotreatment with hemostatic agents on clot parameters. FXIII cotreatment accelerated FXIII activation and crosslinking of fibrin and α2 -antiplasmin. These data provide biochemical rationale for FXIII cotreatment in hemophilia.
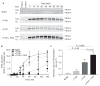
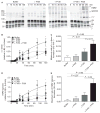
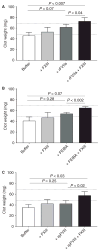
Summary: Background Hemophilia A results from the absence, deficiency or inhibition of factor VIII. Bleeding is treated with hemostatic agents (FVIII, recombinant activated FVII [rFVIIa], anti-inhibitor coagulation complex [FEIBA], or recombinant porcine FVIII [rpFVIII]). Despite treatment, some patients have prolonged bleeding. FXIII-A2 B2 (FXIII) is a protransglutaminase. During clot contraction, thrombin-activated FXIII (FXIIIa) crosslinks fibrin and α2 -antiplasmin, which promotes red blood cell retention and increases clot stability and weight. We hypothesized that FXIII cotreatment in hemophilia would accelerate FXIII activation, leading to increased fibrin crosslinking. Methods FVIII-deficient plasma and whole blood were clotted with or without hemostatic agents (FVIII, rFVIIa, FEIBA, or recombinant B-domain-deleted porcine FVIII [rpFVIII]) and/or FXIII. The effects on FXIII activation, thrombin generation, fibrin and α2 -antiplasmin crosslinking, clot formation and clot weight were measured by western blotting, calibrated automated thrombography, thromboelastography, and clot contraction assays. Results As compared with FVIII-treated hemophilic plasma, FVIII + FXIII cotreatment accelerated FXIIIa formation without increasing thrombin generation. As compared with buffer-treated or FXIII-treated hemophilic plasma, FVIII treatment and FVIII + FXIII cotreatment increased the generation and amount of crosslinked fibrin, including α-chain-rich high molecular weight species and crosslinked α2 -antiplasmin. In the presence of FVIII inhibitors, as compared with hemostatic treatments (rFVIIa, FEIBA, or rpFVIII) alone, FXIII cotreatment increased whole blood clot weight. Conclusion In hemophilia A plasma and whole blood, FXIII cotreatment with hemostatic agents accelerated FXIIIa formation, increased the generation and amount of fibrin α-chain crosslinked species, accelerated α2 -antiplasmin crosslinking, and increased clot weight. FXIII cotreatment with hemostatic therapy may augment hemostasis through increased crosslinking of fibrin and α2 -antiplasmin.
Keywords: factor XIII; fibrin; hemophilia; hemostasis; α2-antiplasmin.
© 2017 International Society on Thrombosis and Haemostasis.
Conflict of interest statement
CSL Behring provided funds to the Hemostasis and Thrombosis Research Society for the Mentored Research Award program, but had no input into the selection of awardees. CSL Behring had no direct interaction with the authors on the study design or results. The authors state that they have no other conflict of interest.
Figures

Similar articles
-
Plasma fibrin clots of pulmonary embolism patients present increased amounts of factor XIII and alpha2-antiplasmin at 3 months' anticoagulation since the acute phase.J Physiol Pharmacol. 2020 Aug;71(4). doi: 10.26402/jpp.2020.4.07. Epub 2020 Nov 15. J Physiol Pharmacol. 2020. PMID: 33214340
-
The role of recombinant factor VIIa (FVIIa) in fibrin structure in the absence of FVIII/FIX.J Thromb Haemost. 2003 Jun;1(6):1215-9. doi: 10.1046/j.1538-7836.2003.00242.x. J Thromb Haemost. 2003. PMID: 12871322
-
Factor XIII combined with recombinant factor VIIa: a new means of treating severe hemophilia A.J Thromb Haemost. 2011 Mar;9(3):510-6. doi: 10.1111/j.1538-7836.2010.04171.x. J Thromb Haemost. 2011. PMID: 21155966 Clinical Trial.
-
Recombinant factor VIIa (eptacog alfa): a review of its use in congenital hemophilia with inhibitors, acquired hemophilia, and other congenital bleeding disorders.BioDrugs. 2008;22(2):121-36. doi: 10.2165/00063030-200822020-00005. BioDrugs. 2008. PMID: 18345709 Review.
-
New insights into the coagulation system and implications for new therapeutic options with recombinant factor VIIa.Curr Med Chem. 2003 May;10(10):797-811. doi: 10.2174/0929867033457728. Curr Med Chem. 2003. PMID: 12678684 Review.
Cited by
-
Mechanistic rationale for factor XIII cotreatment in haemophilia.Haemophilia. 2019 Nov;25(6):e377-e378. doi: 10.1111/hae.13855. Epub 2019 Oct 2. Haemophilia. 2019. PMID: 31577382 Free PMC article. No abstract available.
-
Fibrin(ogen) as a Therapeutic Target: Opportunities and Challenges.Int J Mol Sci. 2021 Jun 28;22(13):6916. doi: 10.3390/ijms22136916. Int J Mol Sci. 2021. PMID: 34203139 Free PMC article. Review.
-
Explore the Staging of Cerebral Venous Thrombosis Through Fibrinolytic Indicators.Clin Appl Thromb Hemost. 2024 Jan-Dec;30:10760296241304777. doi: 10.1177/10760296241304777. Clin Appl Thromb Hemost. 2024. PMID: 39639579 Free PMC article.
-
Inactivation of human plasma alters the structure and biomechanical properties of engineered tissues.Front Bioeng Biotechnol. 2022 Aug 23;10:908250. doi: 10.3389/fbioe.2022.908250. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36082161 Free PMC article.
-
The factor XIII-A Val34Leu polymorphism decreases whole blood clot mass at high fibrinogen concentrations.J Thromb Haemost. 2020 Apr;18(4):885-894. doi: 10.1111/jth.14744. Epub 2020 Feb 28. J Thromb Haemost. 2020. PMID: 31989767 Free PMC article.
References
-
- Cawthern KM, van’t Veer C, Lock JB, Di Lorenzo ME, Branda RF, Mann KG. Blood coagulation in hemophilia A and hemophilia C. Blood. 1998;91:4581–92. - PubMed
-
- Butenas SS, Brummel KE, Branda RF, Paradis SG, Mann KG. Mechanism of factor VIIa-dependent coagulation in hemophilia blood. Blood. 2002;99:923–30. - PubMed
-
- Dargaud Y, Lienhart A, Negrier C. Prospective assessment of thrombin generation test for dose monitoring of bypassing therapy in hemophilia patients with inhibitors undergoing elective surgery. Blood. 2010;116:5734–7. - PubMed
-
- Sixma JJ, van den Berg A. The haemostatic plug in haemophilia A: a morphological study of haemostatic plug formation in bleeding time skin wounds of patients with severe haemophilia A. Br J Haematol. 1984;58:741–53. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

