Model projections on the impact of HCV treatment in the prevention of HCV transmission among people who inject drugs in Europe
- PMID: 29080808
- PMCID: PMC5841161
- DOI: 10.1016/j.jhep.2017.10.010
Model projections on the impact of HCV treatment in the prevention of HCV transmission among people who inject drugs in Europe
Abstract
Background & aims: Prevention of hepatitis C virus (HCV) transmission among people who inject drugs (PWID) is critical for eliminating HCV in Europe. We estimated the impact of current and scaled-up HCV treatment with and without scaling up opioid substitution therapy (OST) and needle and syringe programmes (NSPs) across Europe over the next 10 years.
Methods: We collected data on PWID HCV treatment rates, PWID prevalence, HCV prevalence, OST, and NSP coverage from 11 European settings. We parameterised an HCV transmission model to setting-specific data that project chronic HCV prevalence and incidence among PWID.
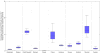
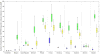
Results: At baseline, chronic HCV prevalence varied from <25% (Slovenia/Czech Republic) to >55% (Finland/Sweden), and <2% (Amsterdam/Hamburg/Norway/Denmark/Sweden) to 5% (Slovenia/Czech Republic) of chronically infected PWID were treated annually. The current treatment rates using new direct-acting antivirals (DAAs) may achieve observable reductions in chronic prevalence (38-63%) in 10 years in Czech Republic, Slovenia, and Amsterdam. Doubling the HCV treatment rates will reduce prevalence in other sites (12-24%; Belgium/Denmark/Hamburg/Norway/Scotland), but is unlikely to reduce prevalence in Sweden and Finland. Scaling-up OST and NSP to 80% coverage with current treatment rates using DAAs could achieve observable reductions in HCV prevalence (18-79%) in all sites. Using DAAs, Slovenia and Amsterdam are projected to reduce incidence to 2 per 100 person years or less in 10 years. Moderate to substantial increases in the current treatment rates are required to achieve the same impact elsewhere, from 1.4 to 3 times (Czech Republic and France), 5-17 times (France, Scotland, Hamburg, Norway, Denmark, Belgium, and Sweden), to 200 times (Finland). Scaling-up OST and NSP coverage to 80% in all sites reduces treatment scale-up needed by 20-80%.
Conclusions: The scale-up of HCV treatment and other interventions is needed in most settings to minimise HCV transmission among PWID in Europe.
Lay summary: Measuring the amount of HCV in the population of PWID is uncertain. To reduce HCV infection to minimal levels in Europe will require scale-up of both HCV treatment and other interventions that reduce injecting risk (especially OST and provision of sterile injecting equipment).
Keywords: Direct-acting antivirals; Hepatitis C; Opioid substitution therapy; PWID.
Copyright © 2017 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Figures


Comment in
-
Achieving hepatitis C elimination in Europe - To treatment scale-up and beyond.J Hepatol. 2018 Mar;68(3):383-385. doi: 10.1016/j.jhep.2017.12.004. Epub 2017 Dec 9. J Hepatol. 2018. PMID: 29233629 No abstract available.
References
-
- Williams R, et al. Addressing liver disease in the UK: a blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. Lancet. 2014;384(9958):1953–97. - PubMed
-
- Ly KN, et al. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Annals of internal medicine. 2012;156(4):271–278. - PubMed
-
- Cowie B, Allard N, MacLachlan J. O86 EUROPEAN RESPONSES IN FOCUS: COMPARING VIRAL HEPATITIS AND HIV RELATED DEATHS IN EUROPE 1990–2010 IN THE GLOBAL BURDEN OF DISEASE STUDY 2010. Journal of Hepatology. 2014;1(60):S35–S36.
-
- ECDC and EMCDDA. Prevention and control of infectious diseases among people who inject drugs. Stockholm: ECDC; 2011. pp. 4–5.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

