Human endoglin as a potential new partner involved in platelet-endothelium interactions
- PMID: 29080903
- PMCID: PMC5843676
- DOI: 10.1007/s00018-017-2694-7
Human endoglin as a potential new partner involved in platelet-endothelium interactions
Abstract
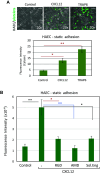
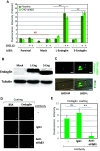
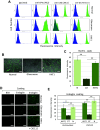
Complex interactions between platelets and activated endothelium occur during the thrombo-inflammatory reaction at sites of vascular injuries and during vascular hemostasis. The endothelial receptor endoglin is involved in inflammation through integrin-mediated leukocyte adhesion and transmigration; and heterozygous mutations in the endoglin gene cause hereditary hemorrhagic telangiectasia type 1. This vascular disease is characterized by a bleeding tendency that is postulated to be a consequence of telangiectasia fragility rather than a platelet defect, since platelets display normal functions in vitro in this condition. Here, we hypothesize that endoglin may act as an adhesion molecule involved in the interaction between endothelial cells and platelets through integrin recognition. We find that the extracellular domain of human endoglin promotes specific platelet adhesion under static conditions and confers resistance of adherent platelets to detachment upon exposure to flow. Also, platelets adhere to confluent endothelial cells in an endoglin-mediated process. Remarkably, Chinese hamster ovary cells ectopically expressing the human αIIbβ3 integrin acquire the capacity to adhere to myoblast transfectants expressing human endoglin, whereas platelets from Glanzmann's thrombasthenia patients lacking the αIIbβ3 integrin are defective for endoglin-dependent adhesion to endothelial cells. Furthermore, the bleeding time, but not the prothrombin time, is significantly prolonged in endoglin-haplodeficient (Eng +/-) mice compared to Eng +/+ animals. These results suggest a new role for endoglin in αIIbβ3 integrin-mediated adhesion of platelets to the endothelium, and may provide a better understanding on the basic cellular mechanisms involved in hemostasis and thrombo-inflammatory events.
Keywords: CXCL12; HHT; Hereditary hemorrhagic telangiectasia; Preeclampsia; RGD; TGF-β.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




Similar articles
-
ADAM 15 is an adhesion receptor for platelet GPIIb-IIIa and induces platelet activation.Thromb Haemost. 2005 Sep;94(3):555-61. doi: 10.1160/TH04-12-0784. Thromb Haemost. 2005. PMID: 16268472
-
Engagement of αIIbβ3 (GPIIb/IIIa) with ανβ3 integrin mediates interaction of melanoma cells with platelets: a connection to hematogenous metastasis.J Biol Chem. 2012 Jan 13;287(3):2168-78. doi: 10.1074/jbc.M111.269811. Epub 2011 Nov 18. J Biol Chem. 2012. PMID: 22102277 Free PMC article.
-
Soluble endoglin reduces thrombus formation and platelet aggregation via interaction with αIIbβ3 integrin.J Thromb Haemost. 2023 Jul;21(7):1943-1956. doi: 10.1016/j.jtha.2023.03.023. Epub 2023 Mar 28. J Thromb Haemost. 2023. PMID: 36990159
-
Endoglin as an Adhesion Molecule in Mature and Progenitor Endothelial Cells: A Function Beyond TGF-β.Front Med (Lausanne). 2019 Jan 30;6:10. doi: 10.3389/fmed.2019.00010. eCollection 2019. Front Med (Lausanne). 2019. PMID: 30761306 Free PMC article. Review.
-
Novel vascular roles of human endoglin in pathophysiology.J Thromb Haemost. 2023 Sep;21(9):2327-2338. doi: 10.1016/j.jtha.2023.06.007. Epub 2023 Jun 12. J Thromb Haemost. 2023. PMID: 37315795 Review.
Cited by
-
Endoglin: An 'Accessory' Receptor Regulating Blood Cell Development and Inflammation.Int J Mol Sci. 2020 Dec 3;21(23):9247. doi: 10.3390/ijms21239247. Int J Mol Sci. 2020. PMID: 33287465 Free PMC article. Review.
-
Syntenin Controls Extracellular Vesicle-Induced Tumour Migration by Regulating the Expression of Adhesion Proteins on Small Extracellular Vesicles.J Extracell Vesicles. 2025 Aug;14(8):e70133. doi: 10.1002/jev2.70133. J Extracell Vesicles. 2025. PMID: 40831280 Free PMC article.
-
The Association of IL-17 and PlGF/sENG Ratio in Pre-Eclampsia and Adverse Pregnancy Outcomes.Int J Environ Res Public Health. 2022 Dec 31;20(1):768. doi: 10.3390/ijerph20010768. Int J Environ Res Public Health. 2022. PMID: 36613090 Free PMC article.
-
The Role of Endoglin in Hepatocellular Carcinoma.Int J Mol Sci. 2021 Mar 22;22(6):3208. doi: 10.3390/ijms22063208. Int J Mol Sci. 2021. PMID: 33809908 Free PMC article. Review.
-
Disseminated intravascular coagulation phenotype is regulated by the TRPM7 channel during sepsis.Biol Res. 2023 Mar 3;56(1):8. doi: 10.1186/s40659-023-00419-4. Biol Res. 2023. PMID: 36869357 Free PMC article.
References
-
- Letarte M, Bourdeau A, Vera S, et al. et al. CD105 workshop panel report. In: Kishimoto T, Kikutani H, von dem Borne AEGKR, et al.et al., editors. Leucocyte typing VI. New York: Garland Publishing Inc.; 1997. pp. 703–708.
-
- Cheifetz S, Bellón T, Calés C, Vera S, Bernabeu C, Massagué J, Letarte M. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J Biol Chem. 1992;267(27):19027–19030. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- SAF2013-43421-R/Ministerio de Economia y Competitividad of Spain/International
- BFU2010-15237/Ministerio de Economia y Competitividad of Spain/International
- SAF2013-45784-R/Ministerio de Economia y Competitividad of Spain/International
- ISCIII-CB06/07/0038/Centro de Investigacion Biomedica en Red de Enfermedades Raras/International
- ER16PIAC707/Centro de Investigacion Biomedica en Red de Enfermedades Raras/International
LinkOut - more resources
Full Text Sources
Other Literature Sources

