Benefit-Risk Assessment of Crataegus Extract WS 1442: An Evidence-Based Review
- PMID: 29080984
- PMCID: PMC5772138
- DOI: 10.1007/s40256-017-0249-9
Benefit-Risk Assessment of Crataegus Extract WS 1442: An Evidence-Based Review
Abstract
Preparations from Crataegus (hawthorn) have a long history in the treatment of heart failure. WS 1442 is a dry extract from hawthorn leaves with flowers (4-6.6:1), extraction solvent of ethanol 45% (w/w), adjusted to 17.3-20.1% of oligomeric procyanidins. Nonclinical studies show that WS 1442 has positive inotropic and antiarrhythmic properties and protects the myocardium from ischemic damage, reperfusion injury, and hypertension-related hypertrophy, improves endothelial functions such as NO synthesis, and delays endothelial senescence. Randomized, controlled trials in patients with heart failure have demonstrated that the herbal medicinal product increases functional capacity, alleviates disabling symptoms, and improves health-related quality of life, all of which have become important targets of heart failure therapy according to current disease management guidelines. Clinical trials (including a 2-year mortality study with polypharmacy and > 1300 patients exposed) and post-marketing surveillance studies have shown that WS 1442 has a very favorable safety profile both as monotherapy and as add-on therapy, where no drug interactions have been observed. No specific adverse reactions to WS 1442 are known to date. WS 1442 may thus help to close the therapeutic gap between systolic and diastolic heart failure for which evidence of efficacy for other cardioactive drugs is sparse. Scientific evidence shows that WS 1442 is safe and has a beneficial effect in patients with heart failure corresponding to New York Heart Association classes II or III. The benefit-risk assessment for WS 1442 is therefore positive.
Conflict of interest statement
Conflict of interest
CJFH, WSC and JE have received honoraria from Dr. Willmar Schwabe GmbH & Co. KG, Karlsruhe, Germany for conducting, and writing for, the SPICE study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the studies.
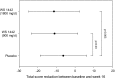
Figures


References
-
- Inman T. Foundation for a new theory and practice of medicine. London: John Churchill; 1860.
-
- Der Weißdorn Kaul R. Botanik, Inhaltsstoffe, Qualitätskontrolle, Pharmakologie, Toxikologie und Klinik. Stuttgart: Wissenschaftliche Verlagsgesellschaft; 1998.
-
- Jennings MC. Crataegus oxyacantha in the treatment of heart disease. N Y Med J. 1896;10(10):491–3.
-
- Bundesgesundheitsamt. Monographie—Crataegi folium cum flore (Weißdornblätter mit Blüten). Bundesanzeiger. 1994;133 (19 July 1994):7360–1.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

