Conformational memory in the association of the transmembrane protein phospholamban with the sarcoplasmic reticulum calcium pump SERCA
- PMID: 29081402
- PMCID: PMC5766962
- DOI: 10.1074/jbc.M117.794453
Conformational memory in the association of the transmembrane protein phospholamban with the sarcoplasmic reticulum calcium pump SERCA
Abstract
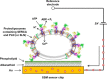
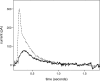
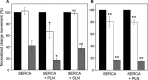
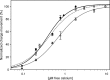
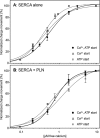
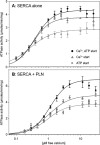
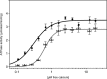
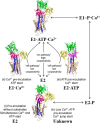
The sarcoplasmic reticulum Ca2+-ATPase SERCA promotes muscle relaxation by pumping calcium ions from the cytoplasm into the sarcoplasmic reticulum. SERCA activity is regulated by a variety of small transmembrane peptides, most notably by phospholamban in cardiac muscle and sarcolipin in skeletal muscle. However, how phospholamban and sarcolipin regulate SERCA is not fully understood. In the present study, we evaluated the effects of phospholamban and sarcolipin on calcium translocation and ATP hydrolysis by SERCA under conditions that mimic environments in sarcoplasmic reticulum membranes. For pre-steady-state current measurements, proteoliposomes containing SERCA and phospholamban or sarcolipin were adsorbed to a solid-supported membrane and activated by substrate concentration jumps. We observed that phospholamban altered ATP-dependent calcium translocation by SERCA within the first transport cycle, whereas sarcolipin did not. Using pre-steady-state charge (calcium) translocation and steady-state ATPase activity under substrate conditions (various calcium and/or ATP concentrations) promoting particular conformational states of SERCA, we found that the effect of phospholamban on SERCA depends on substrate preincubation conditions. Our results also indicated that phospholamban can establish an inhibitory interaction with multiple SERCA conformational states with distinct effects on SERCA's kinetic properties. Moreover, we noted multiple modes of interaction between SERCA and phospholamban and observed that once a particular mode of association is engaged it persists throughout the SERCA transport cycle and multiple turnover events. These observations are consistent with conformational memory in the interaction between SERCA and phospholamban, thus providing insights into the physiological role of phospholamban and its regulatory effect on SERCA transport activity.
Keywords: allosteric regulation; calcium ATPase; calcium translocation; cardiac muscle; membrane transport; phospholamban; sarcolipin; sarcoplasmic reticulum (SR); solid supported membrane.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
References
-
- Toyoshima C. (2009) How Ca2+-ATPase pumps ions across the sarcoplasmic reticulum membrane. Biochim. Biophys. Acta 1793, 941–946 - PubMed
-
- Toyoshima C., and Cornelius F. (2013) New crystal structures of PII-type ATPases: excitement continues. Curr. Opin. Struct. Biol. 23, 507–514 - PubMed
-
- Møller J. V., Olesen C., Winther A. M., and Nissen P. (2010) The sarcoplasmic Ca2+-ATPase: design of a perfect chemi-osmotic pump. Q Rev. Biophys. 43, 501–566 - PubMed
-
- de Meis L., and Vianna A. L. (1979) Energy interconversion by the Ca-dependent ATPase of the sarcoplasmic reticulum. Annu. Rev. Biochem. 48, 275–292 - PubMed
-
- Jones L. R., Cornea R. L., and Chen Z. (2002) Close proximity between residue 30 of phospholamban and cysteine 318 of the cardiac Ca2+ pump revealed by intermolecular thiol cross-linking. J. Biol. Chem. 277, 28319–28329 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

