Isolation and characterization of urinary extracellular vesicles: implications for biomarker discovery
- PMID: 29081510
- PMCID: PMC5941934
- DOI: 10.1038/nrneph.2017.148
Isolation and characterization of urinary extracellular vesicles: implications for biomarker discovery
Abstract
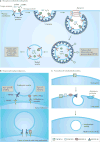
Urine is a valuable diagnostic medium and, with the discovery of urinary extracellular vesicles, is viewed as a dynamic bioactive fluid. Extracellular vesicles are lipid-enclosed structures that can be classified into three categories: exosomes, microvesicles (or ectosomes) and apoptotic bodies. This classification is based on the mechanisms by which membrane vesicles are formed: fusion of multivesicular bodies with the plasma membranes (exosomes), budding of vesicles directly from the plasma membrane (microvesicles) or those shed from dying cells (apoptotic bodies). During their formation, urinary extracellular vesicles incorporate various cell-specific components (proteins, lipids and nucleic acids) that can be transferred to target cells. The rigour needed for comparative studies has fueled the search for optimal approaches for their isolation, purification, and characterization. RNA, the newest extracellular vesicle component to be discovered, has received substantial attention as an extracellular vesicle therapeutic, and compelling evidence suggests that ex vivo manipulation of microRNA composition may have uses in the treatment of kidney disorders. The results of these studies are building the case that urinary extracellular vesicles act as mediators of renal pathophysiology. As the field of extracellular vesicle studies is burgeoning, this Review focuses on primary data obtained from studies of human urine rather than on data from studies of laboratory animals or cultured immortalized cells.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13:269–288. - PubMed
-
- Taylor DD, Doellgast GJ. Quantitation of peroxidase-antibody binding to membrane fragments using column chromatography. Anal Biochem. 1979;98:53–59. - PubMed
-
- Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20:1487–1495. - PubMed
-
- Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

