Precision-guided, Personalized Intrapleural Fibrinolytic Therapy for Empyema and Complicated Parapneumonic Pleural Effusions: The Case for the Fibrinolytic Potential
- PMID: 29081644
- PMCID: PMC5654485
- DOI: 10.1097/CPM.0000000000000216
Precision-guided, Personalized Intrapleural Fibrinolytic Therapy for Empyema and Complicated Parapneumonic Pleural Effusions: The Case for the Fibrinolytic Potential
Abstract
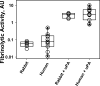
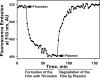
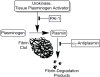
Complicated pleural effusions and empyema with loculation and failed drainage are common clinical problems. In adults, intrapleural fibrinolytic therapy is commonly used with variable results and therapy remains empiric. Despite the intrapleural use of various plasminogen activators; fibrinolysins, for about sixty years, there is no clear consensus about which agent is most effective. Emerging evidence demonstrates that intrapleural administration of plasminogen activators is subject to rapid inhibition by plasminogen activator inhibitor-1 and that processing of fibrinolysins is importantly influenced by other factors including the levels and quality of pleural fluid DNA. Current therapy for loculation that accompanies pleural infections also includes surgery, which is invasive and for which patient selection can be problematic. Most of the clinical literature published to date has used flat dosing of intrapleural fibrinolytic therapy in all subjects but little is known about how that strategy influences the processing of the administered fibrinolysin or how this influences outcomes. We developed a new test of pleural fluids ex vivo, which is called the Fibrinolytic Potential or FP, in which a dose of a fibrinolysin is added to pleural fluids ex vivo after which the fibrinolytic activity is measured and normalized to baseline levels. Testing in preclinical and clinical empyema fluids reveals a wide range of responses, indicating that individual patients will likely respond differently to flat dosing of fibrinolysins. The test remains under development but is envisioned as a guide for dosing of these agents, representing a novel candidate approach to personalization of intrapleural fibrinolytic therapy.
Keywords: Intrapleural fibrinolytic therapy; plasminogen activators and personalized therapy.
Conflict of interest statement
Disclosures: All authors have conflict of interest plans acknowledging and managing these declared conflicts of interest through The University of Texas Health Science Center at Tyler. Dr. R. Idell has a declared Conflict of Interest given his relationship to Dr. S. Idell. The FPA; a test of pleural fluid that may predict outcomes of fibrinolytic therapy, guide dosing and dosing intervals. (GF, SI, AK) appl # 15/086,623, Notice of publication 10.2016. Dr. Rahman received an unrestricted educational grant from Roche UK in support of the MIST2 study. Kathy Koenig, Torry Tucker and Ali Azghani have no conflicts. All human and animal studies described in this manuscript were approved by the UTHSCT Human Subjects Institutional Review Board and Institutional Animal Care and Utilization Committees, respectively.
Figures


References
-
- Corcoran JP, Hallifax R, Rahman NM. New therapeutic approaches to pleural infection. Curr Opin Infect Dis. 2013;26:196–202. - PubMed
-
- Rahman NM, Maskell NA, West A, Teoh R, Arnold A, Mackinlay C, Peckham D, Davies CW, Ali N, Kinnear W, Bentley A, Kahan BC, Wrightson JM, Davies HE, Hooper CE, Lee YC, Hedley EL, Crosthwaite N, Choo L, Helm EJ, Gleeson FV, Nunn AJ, Davies RJ. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med. 2011;365:518–26. - PubMed
-
- Sonnappa S, Cohen G, Owens CM, van Doorn C, Cairns J, Stanojevic S, Elliott MJ, Jaffe A. Comparison of Urokinase and Video-assisted Thoracoscopic Surgery for Treatment of Childhood Empyema. Am J Respir Crit Care Med. 2006;174:221–7. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
