A β-Carbon elimination strategy for convenient in situ access to cyclopentadienyl metal complexes
- PMID: 29081949
- PMCID: PMC5635420
- DOI: 10.1039/c7sc02986a
A β-Carbon elimination strategy for convenient in situ access to cyclopentadienyl metal complexes
Abstract
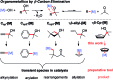
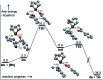
The electronic and steric properties of tailored cyclopentadienyl (Cp) ligands are powerful handles to modulate the catalytic properties of their metal complexes. This requires the individual preparation, purification and storage of each ligand/metal combination. Alternative, ideally in situ, complexation protocols would be of high utility. We disclose a new approach to access Cp metal complexes. Common metal precursors rapidly react with cyclopentadienyl carbinols via β-carbon eliminations to directly give the Cp-metal complexes. An advantage of this is the direct and flexible use of storable pre-ligands. No auxiliary base is required and the Cp complexes can be prepared in situ in the reaction vessel for subsequent catalytic transformations.
Figures



References
-
- Hartwig J., Organotransition Metal Chemistry: From Bonding to Catalysis, University Science Books, Sausalito, CA, 2010.
-
- Davis T. A., Wang C., Rovis T. Synlett. 2015;26:1520. - PMC - PubMed
- Piou T., Rovis T. J. Am. Chem. Soc. 2014;136:11292. - PMC - PubMed
- Piou T., Rovis T. Nature. 2015;527:86. - PMC - PubMed
- Romanov-Michailidis F., Sedillo K. F., Neely J. M., Rovis T. J. Am. Chem. Soc. 2015;137:8892. - PMC - PubMed
- Hoshino Y., Shibata Y., Tanaka K. Adv. Synth. Catal. 2014;356:1577.
- Hyster T. K., Rovis T. Chem. Commun. 2011;47:11846. - PMC - PubMed
- Shibata Y., Tanaka K. Angew. Chem., Int. Ed. 2011;50:10917. - PubMed
-
- Hyster T. K., Dalton D. M., Rovis T. Chem. Sci. 2015;6:254. - PMC - PubMed
- Hyster T. K., Rovis T. Chem. Sci. 2011;2:1606. - PMC - PubMed
- Neely J. M., Rovis T. J. Am. Chem. Soc. 2014;136:2735. - PMC - PubMed
- Wodrich M. D., Ye B., Gonthier J. F., Corminboeuf C., Cramer N. Chem. –Eur. J. 2014;20:15409. - PubMed
-
-
For a recent review see:
- Newton C. G., Kossler D., Cramer N. J. Am. Chem. Soc. 2016;138:3935. - PubMed
- Ye B., Cramer N. Acc. Chem. Res. 2015;48:1308. - PubMed
- Erker G., van der Zeijden A. A. H. Angew. Chem., Int. Ed. 1990;29:512.
- Ye B., Cramer N. Science. 2012;338:504. - PubMed
- Hyster T. K., Knorr L., Ward T. R., Rovis T. Science. 2012;338:500. - PMC - PubMed
- Ye B., Cramer N. J. Am. Chem. Soc. 2013;135:636. - PubMed
- Kossler D., Cramer N. J. Am. Chem. Soc. 2015;137:12478. - PubMed
- Song G., Wylie W. N. O., Hou Z. J. Am. Chem. Soc. 2014;136:12209. - PubMed
- Dieckmann M., Jang Y.-S., Cramer N. Angew. Chem., Int. Ed. 2015;127:12317. - PubMed
-
-
- Dubois R., US4962253 A, The Dow Chemical, 1990.
- King R. B. Inorg. Chem. 1963;2:528.
- Rausch M. D., Genetti R. A. J. Org. Chem. 1970;35:3888.
- Weding N., Jackstell R., Jiao H., Spannenberg A., Hapke M. Adv. Synth. Catal. 2011;353:3423.
- White C., Yates A., Maitlis P. M. and Heinekey D. M., in Inorg. Synth., John Wiley & Sons, Inc., 2007, p. 228.
- Chen H., Hartwig J. and Semple T. C., WO 01/64689 A1, Shell Internationale Research Maatschappij B.V., Yale University, 2001.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

