A unified photoredox-catalysis strategy for C(sp3)-H hydroxylation and amidation using hypervalent iodine
- PMID: 29081950
- PMCID: PMC5635418
- DOI: 10.1039/c7sc02773g
A unified photoredox-catalysis strategy for C(sp3)-H hydroxylation and amidation using hypervalent iodine
Abstract
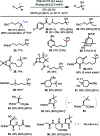
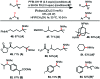
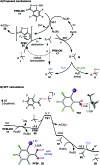
We report a unified photoredox-catalysis strategy for both hydroxylation and amidation of tertiary and benzylic C-H bonds. Use of hydroxyl perfluorobenziodoxole (PFBl-OH) oxidant is critical for efficient tertiary C-H functionalization, likely due to the enhanced electrophilicity of the benziodoxole radical. Benzylic methylene C-H bonds can be hydroxylated or amidated using unmodified hydroxyl benziodoxole oxidant Bl-OH under similar conditions. An ionic mechanism involving nucleophilic trapping of a carbocation intermediate by H2O or CH3CN cosolvent is presented.
Figures


References
-
-
For selected metal-catalyzed C(sp3)–H oxygenation reactions, see:
- Chen M. S., White M. C. Science. 2007;318:783. - PubMed
- Zhang Y.-H., Yu J.-Q. J. Am. Chem. Soc. 2009;131:14654. - PubMed
- Zhou M., Schley N. D., Crabtree R. H. J. Am. Chem. Soc. 2010;132:12550. - PubMed
- McNeill E., Du Bois J. J. Am. Chem. Soc. 2010;132:10202. - PubMed
- Lee M., Sanford M. S. J. Am. Chem. Soc. 2015;137:12796. - PMC - PubMed
-
-
-
For selected C–H hydroxylation with dioxiranes and oxaziridines, see:
- Yang D., Wong M.-K., Wang X.-C., Tang Y.-C. J. Am. Chem. Soc. 1998;120:6611.
- Brodsky B. H., Du Bois J. J. Am. Chem. Soc. 2005;127:15391. - PubMed
- Chen K., Richter J. M., Baran P. S. J. Am. Chem. Soc. 2008;130:7247. - PubMed
- Litvinas N. D., Brodsky B. H., Du Bois J. Angew. Chem., Int. Ed. 2009;48:4513. - PubMed
- Kasuya S., Kamijo S., Inoue M. Org. Lett. 2009;11:3630. - PubMed
- Adams A. M., Du Bois J. Chem. Sci. 2014;5:656.
-
LinkOut - more resources
Full Text Sources
Other Literature Sources

