Initiative for Molecular Profiling and Advanced Cancer Therapy (IMPACT): An MD Anderson Precision Medicine Study
- PMID: 29082359
- PMCID: PMC5659750
- DOI: 10.1200/PO.17.00002
Initiative for Molecular Profiling and Advanced Cancer Therapy (IMPACT): An MD Anderson Precision Medicine Study
Abstract
Purpose: Genomic profiling is increasingly used in the management of cancer. We have previously reported preliminary results of our precision medicine program. Here, we present response and survival outcomes for 637 additional patients who were referred for phase I trials and were treated with matched targeted therapy (MTT) when available.
Patients and methods: Patients with advanced cancer who underwent tumor genomic analyses were treated with MTT when available.
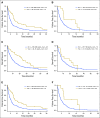
Results: Overall, 1,179 (82.1%) of 1,436 patients had one or more alterations (median age, 59.7 years; men, 41.2%); 637 had one or more actionable aberrations and were treated with MTT (n = 390) or non-MTT (n = 247). Patients who were treated with MTT had higher rates of complete and partial response (11% v 5%; P = .0099), longer failure-free survival (FFS; 3.4 v 2.9 months; P = .0015), and longer overall survival (OS; 8.4 v 7.3 months; P = .041) than did unmatched patients. Two-month landmark analyses showed that, for MTT patients, FFS for responders versus nonresponders was 7.6 versus 4.3 months (P < .001) and OS was 23.4 versus 8.5 months (P < .001), whereas for non-MTT patients (responders v nonresponders), FFS was 6.6 versus 4.1 months (P = .001) and OS was 15.2 versus 7.5 months (P = .43). Patients with phosphatidylinositol 3-kinase (PI3K) and mitogen-activated protein kinase pathway alterations matched to PI3K/Akt/mammalian target of rapamycin axis inhibitors alone demonstrated outcomes comparable to unmatched patients.
Conclusion: Our results support the use of genomic matching. Subset analyses indicate that matching patients who harbor a PI3K and mitogen-activated protein kinase pathway alteration to only a PI3K pathway inhibitor does not improve outcome. We have initiated IMPACT2, a randomized trial to compare treatment with and without genomic selection.
Conflict of interest statement
Apostolia-Maria Tsimberidou
David S. Hong
Yang Ye
No relationship to disclose
Carrie Cartwright
No relationship to disclose
Jennifer J. Wheler
No relationship to disclose
Gerald S. Falchook
Aung Naing
Siqing Fu
No relationship to disclose
Sarina Piha-Paul
Filip Janku
Funda Meric-Bernstam
Patrick Hwu
Bryan Kee
Merrill S. Kies
No relationship to disclose
Russell Broaddus
No relationship to disclose
John Mendelsohn
Kenneth R. Hess
Razelle Kurzrock
Figures






References
-
- Chin L, Andersen JN, Futreal PA: Cancer genomics: From discovery science to personalized medicine. Nat Med 17:297-303, 2011 - PubMed
-
- Druker BJ, Talpaz M, Resta DJ, et al. : Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med 344:1031-1037, 2001 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

