Evaluating Metabolite-Related DNA Oxidation and Adduct Damage from Aryl Amines Using a Microfluidic ECL Array
- PMID: 29083162
- PMCID: PMC5777145
- DOI: 10.1021/acs.analchem.7b03528
Evaluating Metabolite-Related DNA Oxidation and Adduct Damage from Aryl Amines Using a Microfluidic ECL Array
Abstract
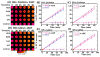
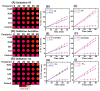
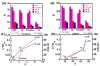
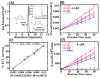
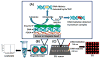
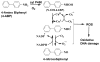
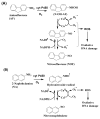
Damage to DNA from the metabolites of drugs and pollutants constitutes a major human toxicity pathway known as genotoxicity. Metabolites can react with metal ions and NADPH to oxidize DNA or participate in SN2 reactions to form covalently linked adducts with DNA bases. Guanines are the main DNA oxidation sites, and 8-oxo-7,8-dihydro-2-deoxyguanosine (8-oxodG) is the initial product. Here we describe a novel electrochemiluminescent (ECL) microwell array that produces metabolites from test compounds and measures relative rates of DNA oxidation and DNA adduct damage. In this new array, films of DNA, metabolic enzymes, and an ECL metallopolymer or complex assembled in microwells on a pyrolytic graphite wafer are housed in dual microfluidic chambers. As reactant solution passes over the wells, metabolites form and can react with DNA in the films to form DNA adducts. These adducts are detected by ECL from a RuPVP polymer that uses DNA as a coreactant. Aryl amines also combine with Cu2+ and NADPH to form reactive oxygen species (ROS) that oxidize DNA. The resulting 8-oxodG was detected selectively by ECL-generating bis(2,2'-bipyridine)-(4-(1,10-phenanthrolin-6-yl)-benzoic acid)Os(II). DNA/enzyme films on magnetic beads were oxidized similarly, and 8-oxodG determined by LC/MS/MS enabled array standardization. The array limit of detection for oxidation was 720 8-oxodG per 106 nucleobases. For a series of aryl amines, metabolite-generated DNA oxidation and adduct formation turnover rates from the array correlated very well with rodent 1/TD50 and Comet assay results.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
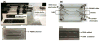
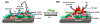
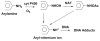

References
-
- Park BK, Boobis A, Clarke S, Goldring CEP, Jones D, Kenna JG, Lambert C, Laverty HG, Naisbitt DJ, Nelson S, Nicoll-Griffith DA, Obach RS, Routledge P, Smith DA, Tweedie DJ, Vermeulen N, Williams DP, Wilson ID, Baillie TA. Nat Rev Drug Discovery. 2011;10:292–307. - PubMed
-
- Farmer PB, Brown K, Tompkins E, Emms VL, Jones DJ, Singh R, Phillips DH. Toxicol Appl Pharmacol. 2005;207:293–301. - PubMed
-
- Spencer WA, Vadhanam MV, Jeyabalan J, Gupta RC. Chem Res Toxicol. 2012;25:305–314. - PubMed
-
- Murata M, Kawanishi S Front. Biosci, Landmark Ed. 2011;16:1132–1143. - PubMed
-
- Bowes J, Brown AJ, Hamon J, Jarolimek W, Sridhar A, Waldron G, Whitebread S. Nat Rev Drug Discovery. 2012;11:909–922. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

