GLIS3 is indispensable for TSH/TSHR-dependent thyroid hormone biosynthesis and follicular cell proliferation
- PMID: 29083325
- PMCID: PMC5707155
- DOI: 10.1172/JCI94417
GLIS3 is indispensable for TSH/TSHR-dependent thyroid hormone biosynthesis and follicular cell proliferation
Abstract
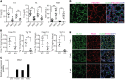
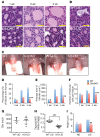
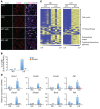
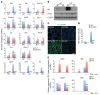
Deficiency in Krüppel-like zinc finger transcription factor GLI-similar 3 (GLIS3) in humans is associated with the development of congenital hypothyroidism. However, the functions of GLIS3 in the thyroid gland and the mechanism by which GLIS3 dysfunction causes hypothyroidism are unknown. In the current study, we demonstrate that GLIS3 acts downstream of thyroid-stimulating hormone (TSH) and TSH receptor (TSHR) and is indispensable for TSH/TSHR-mediated proliferation of thyroid follicular cells and biosynthesis of thyroid hormone. Using ChIP-Seq and promoter analysis, we demonstrate that GLIS3 is critical for the transcriptional activation of several genes required for thyroid hormone biosynthesis, including the iodide transporters Nis and Pds, both of which showed enhanced GLIS3 binding at their promoters. The repression of cell proliferation of GLIS3-deficient thyroid follicular cells was due to the inhibition of TSH-mediated activation of the mTOR complex 1/ribosomal protein S6 (mTORC1/RPS6) pathway as well as the reduced expression of several cell division-related genes regulated directly by GLIS3. Consequently, GLIS3 deficiency in a murine model prevented the development of goiter as well as the induction of inflammatory and fibrotic genes during chronic elevation of circulating TSH. Our study identifies GLIS3 as a key regulator of TSH/TSHR-mediated thyroid hormone biosynthesis and proliferation of thyroid follicular cells and uncovers a mechanism by which GLIS3 deficiency causes neonatal hypothyroidism and prevents goiter development.
Keywords: Endocrinology; Thyroid disease.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

