TrpA1 activation in peripheral sensory neurons underlies the ionic basis of pain hypersensitivity in response to vinca alkaloids
- PMID: 29084244
- PMCID: PMC5662086
- DOI: 10.1371/journal.pone.0186888
TrpA1 activation in peripheral sensory neurons underlies the ionic basis of pain hypersensitivity in response to vinca alkaloids
Abstract
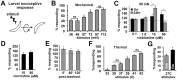
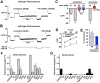
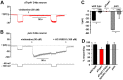
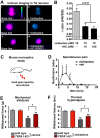
Chemotherapy induced peripheral neuropathy (CIPN), a side effect of many anti-cancer drugs including the vinca alkaloids, is characterized by a severe pain syndrome that compromises treatment in many patients. Currently there are no effective treatments for this pain syndrome except for the reduction of anti-cancer drug dose. Existing data supports the model that the pain associated with CIPN is the result of anti-cancer drugs augmenting the function of the peripheral sensory nociceptors but the cellular mechanisms underlying the effects of anti-cancer drugs on sensory neuron function are not well described. Studies from animal models have suggested a number of disease etiologies including mitotoxicity, axonal degeneration, immune signaling, and reduced sensory innervations but these outcomes are the result of prolonged treatment paradigms and do not necessarily represent the early formative events associated with CIPN. Here we show that acute exposure to vinca alkaloids results in an immediate pain syndrome in both flies and mice. Furthermore, we demonstrate that exposure of isolated sensory neurons to vinca alkaloids results in the generation of an inward sodium current capable of depolarizing these neurons to threshold resulting in neuronal firing. These neuronal effects of vinca alkaloids require the transient receptor potential ankyrin-1 (TrpA1) channel, and the hypersensitization to painful stimuli in response to the acute exposure to vinca alkaloids is reduced in TrpA1 mutant flies and mice. These findings demonstrate the direct excitation of sensory neurons by CIPN-causing chemotherapy drugs, and identify TrpA1 as an important target during the pathogenesis of CIPN.
Conflict of interest statement
Figures



References
-
- Malik B, Stillman M. Chemotherapy-induced peripheral neuropathy. Curr Neurol Neurosci Rep. Current Science Inc; 2008;8: 56–65. doi: 10.1007/s11910-008-0010-5 - DOI - PubMed
-
- Boyette-Davis JA, Walters ET, Dougherty PM. Mechanisms involved in the development of chemotherapy-induced neuropathy. Pain Management. 2015;5: 285–296. doi: 10.2217/pmt.15.19 - DOI - PMC - PubMed
-
- Jaggi AS, Singh N. Mechanisms in cancer-chemotherapeutic drugs-induced peripheral neuropathy. Toxicology. 2012;291: 1–9. doi: 10.1016/j.tox.2011.10.019 - DOI - PubMed
-
- Bhattacharya MRC, Gerdts J, Naylor SA, Royse EX, Ebstein SY, Sasaki Y, et al. A Model of Toxic Neuropathy in Drosophila Reveals a Role for MORN4 in Promoting Axonal Degeneration. Journal of Neuroscience. 2012;32: 5054–5061. doi: 10.1523/JNEUROSCI.4951-11.2012 - DOI - PMC - PubMed
-
- Nieto FR, Entrena JM, Cendán CM, Pozo ED, Vela JM, Baeyens JM. Tetrodotoxin inhibits the development and expression of neuropathic pain induced by paclitaxel in mice. Pain. 2008;137: 520–531. doi: 10.1016/j.pain.2007.10.012 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

