The effect of a novel extracorporeal cytokine hemoadsorption device on IL-6 elimination in septic patients: A randomized controlled trial
- PMID: 29084247
- PMCID: PMC5662220
- DOI: 10.1371/journal.pone.0187015
The effect of a novel extracorporeal cytokine hemoadsorption device on IL-6 elimination in septic patients: A randomized controlled trial
Abstract
Objective: We report on the effect of hemoadsorption therapy to reduce cytokines in septic patients with respiratory failure.
Methods: This was a randomized, controlled, open-label, multicenter trial. Mechanically ventilated patients with severe sepsis or septic shock and acute lung injury or acute respiratory distress syndrome were eligible for study inclusion. Patients were randomly assigned to either therapy with CytoSorb hemoperfusion for 6 hours per day for up to 7 consecutive days (treatment), or no hemoperfusion (control). Primary outcome was change in normalized IL-6-serum concentrations during study day 1 and 7.
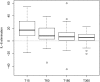
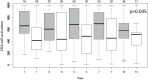
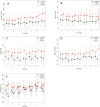
Results: 97 of the 100 randomized patients were analyzed. We were not able to detect differences in systemic plasma IL-6 levels between the two groups (n = 75; p = 0.15). Significant IL-6 elimination, averaging between 5 and 18% per blood pass throughout the entire treatment period was recorded. In the unadjusted analysis, 60-day-mortality was significantly higher in the treatment group (44.7%) compared to the control group (26.0%; p = 0.039). The proportion of patients receiving renal replacement therapy at the time of enrollment was higher in the treatment group (31.9%) when compared to the control group (16.3%). After adjustment for patient morbidity and baseline imbalances, no association of hemoperfusion with mortality was found (p = 0.19).
Conclusions: In this patient population with predominantly septic shock and multiple organ failure, hemoadsorption removed IL-6 but this did not lead to lower plasma IL-6-levels. We did not detect statistically significant differences in the secondary outcomes multiple organ dysfunction score, ventilation time and time course of oxygenation.
Conflict of interest statement
Figures


Comment in
-
Hemoadsorption in critically ill patients with or without COVID-19: A word of caution.J Crit Care. 2021 Oct;65:140-141. doi: 10.1016/j.jcrc.2021.06.007. Epub 2021 Jun 12. J Crit Care. 2021. PMID: 34148009 Free PMC article. No abstract available.
References
-
- Bone RC, Fisher CJ Jr., Clemmer TP, Slotman GJ, Metz CA, Balk RA. Sepsis syndrome: a valid clinical entity. Crit Care Med. 1989;17(5):389–93. . - PubMed
-
- Kellum JA, Kong L, Fink MP, Weissfeld LA, Yealy DM, Pinsky MR, et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med. 2007;167(15):1655–63. doi: 10.1001/archinte.167.15.1655 . - DOI - PMC - PubMed
-
- De Vriese AS, Colardyn FA, Philippe JJ, Vanholder RC, De Sutter JH, Lameire NH. Cytokine removal during continuous hemofiltration in septic patients. J Am Soc Nephrol. 1999;10(4):846–53. . - PubMed
-
- Payen D, Mateo J, Cavaillon JM, Fraisse F, Floriot C, Vicaut E, et al. Impact of continuous venovenous hemofiltration on organ failure during the early phase of severe sepsis: a randomized controlled trial. Crit Care Med. 2009;37(3):803–10. doi: 10.1097/CCM.0b013e3181962316 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

