SIRT1 Controls Acetaminophen Hepatotoxicity by Modulating Inflammation and Oxidative Stress
- PMID: 29084443
- PMCID: PMC9545809
- DOI: 10.1089/ars.2017.7373
SIRT1 Controls Acetaminophen Hepatotoxicity by Modulating Inflammation and Oxidative Stress
Abstract
Aims: Sirtuin 1 (SIRT1) is a key player in liver physiology and a therapeutic target against hepatic inflammation. We evaluated the role of SIRT1 in the proinflammatory context and oxidative stress during acetaminophen (APAP)-mediated hepatotoxicity.
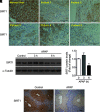
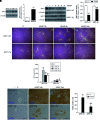
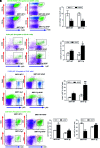
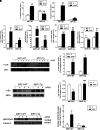
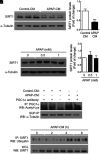
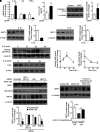
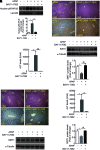
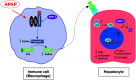
Results: SIRT1 protein levels decreased in human and mouse livers following APAP overdose. SIRT1-Tg mice maintained higher levels of SIRT1 on APAP injection than wild-type mice and were protected against hepatotoxicity by modulation of antioxidant systems and restrained inflammatory responses, with decreased oxidative stress, proinflammatory cytokine messenger RNA levels, nuclear factor kappa B (NFκB) signaling, and cell death. Mouse hepatocytes stimulated with conditioned medium of APAP-treated macrophages (APAP-CM) showed decreased SIRT1 levels; an effect mimicked by interleukin (IL)1β, an activator of NFκB. This negative modulation was abolished by neutralizing IL1β in APAP-CM or silencing p65-NFκB in hepatocytes. APAP-CM of macrophages from SIRT1-Tg mice failed to downregulate SIRT1 protein levels in hepatocytes. In vivo administration of the NFκB inhibitor BAY 11-7082 preserved SIRT1 levels and protected from APAP-mediated hepatotoxicity.
Innovation: Our work evidenced the unique role of SIRT1 in APAP hepatoprotection by targeting oxidative stress and inflammation.
Conclusion: SIRT1 protein levels are downregulated by IL1β/NFκB signaling in APAP hepatotoxicity, resulting in inflammation and oxidative stress. Thus, maintenance of SIRT1 during APAP overdose by inhibiting NFκB might be clinically relevant. Rebound Track: This work was rejected during standard peer review and rescued by Rebound Peer Review (Antioxid Redox Signal 16:293-296, 2012) with the following serving as open reviewers: Rafael de Cabo, Joaquim Ros, Kalervo Hiltunen, and Neil Kaplowitz. Antioxid. Redox Signal. 28, 1187-1208.
Keywords: SIRT1; antioxidant defense; hepatotoxicity; inflammation; interleukin 1β; oxidative stress; paracetamol.
Conflict of interest statement
No competing financial interests exist.
Figures


References
-
- Antoniades CG, Quaglia A, Taams LS, Mitry RR, Hussain M, Abeles R, Possamai LA, Bruce M, McPhail M, Starling C, Wagner B, Barnardo A, Pomplun S, Auzinger G, Bernal W, Heaton N, Vergani D, Thursz MR, and Wendon J. Source and characterization of hepatic macrophages in acetaminophen-induced acute liver failure in humans. Hepatology 56: 735–746, 2012. - PubMed
-
- Barbieri SS, Amadio P, Gianellini S, Tarantino E, Zacchi E, Veglia F, Howe LR, Weksler BB, Mussoni L, and Tremoli E. Cyclooxygenase-2-derived prostacyclin regulates arterial thrombus formation by suppressing tissue factor in a sirtuin-1-dependent-manner. Circulation 126: 1373–1384, 2012. - PubMed
-
- Blazka ME, Elwell MR, Holladay SD, Wilson RE, and Luster MI. Histopathology of acetaminophen-induced liver changes: role of interleukin 1 alpha and tumor necrosis factor alpha. Toxicol Pathol 24: 181–189, 1996. - PubMed
-
- Cavar I, Kelava T, Heinzel R, and Culo F. The role of prostacyclin in modifying acute hepatotoxicity of acetaminophen in mice. Coll Antropol 33(Suppl 2): 25–29, 2009. - PubMed
-
- Cover C, Liu J, Farhood A, Malle E, Waalkes MP, Bajt ML, and Jaeschke H. Pathophysiological role of the acute inflammatory response during acetaminophen hepatotoxicity. Toxicol Appl Pharmacol 216: 98–107, 2006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

