Vitamin D3 repletion versus placebo as adjunctive treatment of heart failure patient quality of life and hormonal indices: a randomized, double-blind, placebo-controlled trial
- PMID: 29084522
- PMCID: PMC5663043
- DOI: 10.1186/s12872-017-0707-y
Vitamin D3 repletion versus placebo as adjunctive treatment of heart failure patient quality of life and hormonal indices: a randomized, double-blind, placebo-controlled trial
Abstract
Background: Vitamin D status may influence heart failure (HF) patient outcomes by affecting b-type natriuretic peptide (BNP), parathyroid hormone (PTH), and enhancing cardiac contractility. Vitamin D deficiency is associated with morbidity and mortality in HF patients. The objective of this study was to determine if vitamin D3 at a comparatively high dose would replete 25-hydroxyvitamin D (25(OH)D) stores, improve BNP, PTH, cardiopulmonary function, reduce inflammatory markers, and improve quality of life (QOL) in HF patients.
Methods: This was a 6 month, parallel group, double-blind, placebo-controlled, single clinic center, randomized trial of supplemental vitamin D3 using a dose of 10,000 IU daily or placebo in 40 vitamin D deficient or insufficient (25(OH)D level ≤ 32 ng/ml) patients with stable New York Heart Association Class II-III HF in a specialty cardiology clinic. All variables were measured at baseline and 6 months. Values between the two treatment groups were assessed using Student's t-test or Mann-Whitney Test. Univariate analysis of covariance was conducted to adjust for variance in baseline 25(OH)D.
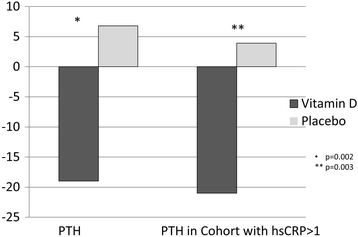
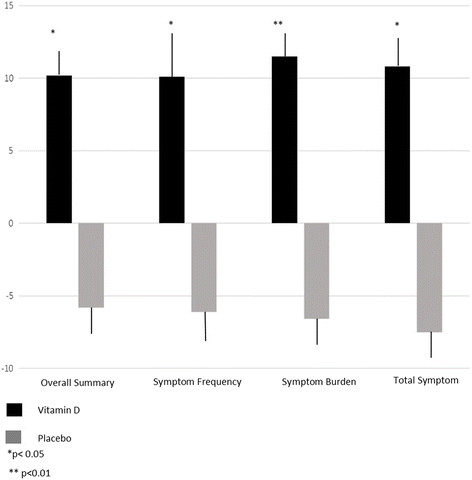
Results: All results were adjusted for baseline 25(OH)D. The change in BNP from baseline was ∆ +30 ± 950 pg/ml for treatment vs. placebo ∆ +400 ± 1900 pg/ml, p = 0.003. 25(OH)D serum levels rose by 49 ± 32 ng/ml in the treatment group vs 4 ± 10 ng/ml in the placebo group, p < 0.001. PTH and exercise chronotropic response index improved in the treatment group vs placebo group, respectively, but both were attenuated by adjustment ((∆-20 ± 20 pg/ml vs ∆ + 7 ± 53 pg/ml respectively (p = 0.01, adjusted p = 0.07)) and (∆ + 0.13 ± 0.26 vs. ∆-0.03 ± 02.9 respectively, p < 0.01, adjusted p = 0.17)). Other measured cardiopulmonary parameters remained unchanged. High sensitivity C-reactive protein (hsCRP) remained unchanged for women, but improved for men (∆-2 ± 4 treatment versus ∆2 ± 5 mg/L placebo, p = 0.05). QOL scores, including composite overall and clinical summary scores significantly improved in treatment compared to placebo (∆ + 10 ± 15 versus -6 ± 15, p < 0.01 and ∆ + 8 ± 14 versus -8 ± 18, p = 0.01, respectively).
Conclusions: Repletion of 25(OH)D may improve QOL in HF patients and may help to normalize BNP, PTH, and hsCRP.
Trial registration: Clinicaltrials.gov, Trial Registration Number: NCT01636570 , First registered 3 July 2012.
Keywords: 25-hydroxyvitamin D; B-type natriuretic peptide; C-reactive protein; Calcitriol; Heart failure; Inflammation; Parathyroid hormone; Quality of life; Vitamin D.
Conflict of interest statement
Ethics approval and consent to participate
All patients provided written, informed consent to participate in this research project. This study was performed in accordance with the Declaration of Helsinki and its amendments. The trial was approved by the Providence Saint Patrick Hospital Institutional Review Board August 8, 2012 (reference number not applicable).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Deda L, Yeshahay Y, Suds S, Cuerden M, Cerrney D, Sochett E et al. Improvements in peripheral vascular function with vitamin D treatment in deficienct adolescents with Type 1 Diabetes. Pediatr Diabetes. 2017. doi:10.1111/pedi.12595. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

