Protein corona: implications for nanoparticle interactions with pulmonary cells
- PMID: 29084556
- PMCID: PMC5663074
- DOI: 10.1186/s12989-017-0223-3
Protein corona: implications for nanoparticle interactions with pulmonary cells
Abstract
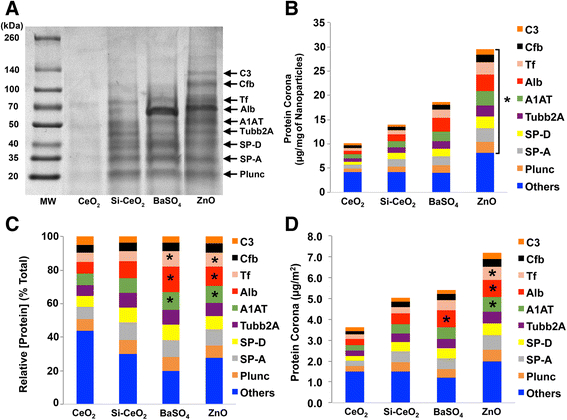
Background: We previously showed that cerium oxide (CeO2), barium sulfate (BaSO4) and zinc oxide (ZnO) nanoparticles (NPs) exhibited different lung toxicity and pulmonary clearance in rats. We hypothesize that these NPs acquire coronas with different protein compositions that may influence their clearance from the lungs.
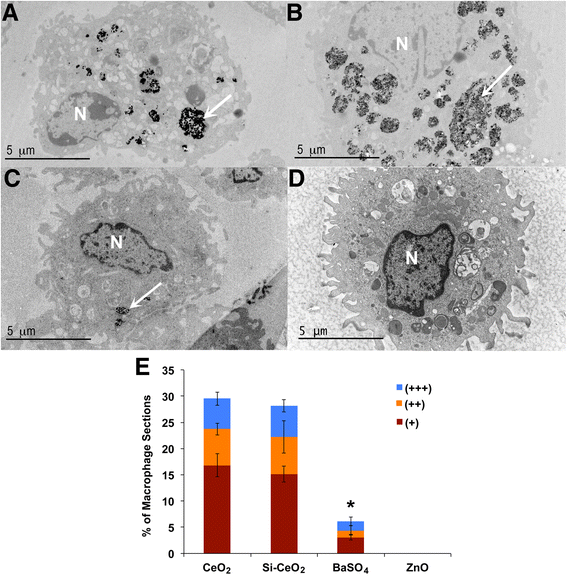
Methods: CeO2, silica-coated CeO2, BaSO4, and ZnO NPs were incubated in rat lung lining fluid in vitro. Then, gel electrophoresis followed by quantitative mass spectrometry was used to characterize the adsorbed proteins stripped from these NPs. We also measured uptake of instilled NPs by alveolar macrophages (AMs) in rat lungs using electron microscopy. Finally, we tested whether coating of gold NPs with albumin would alter their lung clearance in rats.
Results: We found that the amounts of nine proteins in the coronas formed on the four NPs varied significantly. The amounts of albumin, transferrin and α-1 antitrypsin were greater in the coronas of BaSO4 and ZnO than that of the two CeO2 NPs. The uptake of BaSO4 in AMs was less than CeO2 and silica-coated CeO2 NPs. No identifiable ZnO NPs were observed in AMs. Gold NPs coated with albumin or citrate instilled into the lungs of rats acquired the similar protein coronas and were cleared from the lungs to the same extent.
Conclusions: We show that different NPs variably adsorb proteins from the lung lining fluid. The amount of albumin in the NP corona varies as does NP uptake by AMs. However, albumin coating does not affect the translocation of gold NPs across the air-blood barrier. A more extensive database of corona composition of a diverse NP library will develop a platform to help predict the effects and biokinetics of inhaled NPs.
Keywords: Biokinetics; Engineered nanoparticles; Lung macrophage; Nanotoxicity; Protein corona.
Conflict of interest statement
Ethics approval
All animal experiments are in compliance with protocols approved by the Harvard Medical Area Animal Care and Use Committee (Boston, MA).
Consent for publication
All authors read, corrected, and approved the manuscript.
Competing interests
The authors declare no competing financial interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





References
-
- Pirela SV, Lu X, Miousse I, Sisler JD, Qian Y, Guo N, et al. Effects of intratracheally instilled laser printer-emitted engineered nanoparticles in a mouse model: a case study of toxicological implications from nanomaterials released during consumer use. NanoImpact. 2016;1:1–8. doi: 10.1016/j.impact.2015.12.001. - DOI - PMC - PubMed
-
- Servin A, White JC. Nanotechnology in agriculture: next steps for understanding engineered nanoparticle exposure and risk. NanoImpact. 2016;1:9–12. doi: 10.1016/j.impact.2015.12.002. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

