SCORE: Shared care of Colorectal cancer survivors: protocol for a randomised controlled trial
- PMID: 29084595
- PMCID: PMC5663101
- DOI: 10.1186/s13063-017-2245-4
SCORE: Shared care of Colorectal cancer survivors: protocol for a randomised controlled trial
Abstract
Background: Colorectal cancer (CRC) is the most common cancer affecting both men and women. Survivors of CRC often experience various physical and psychological effects arising from CRC and its treatment. These effects may last for many years and adversely affect QoL, and they may not be adequately addressed by standard specialist-based follow-up. Optimal management of these effects should harness the expertise of both primary care and specialist care. Shared models of care (involving both the patient's primary care physician [PCP] and specialist) have the potential to better support survivors and enhance health system efficiency.
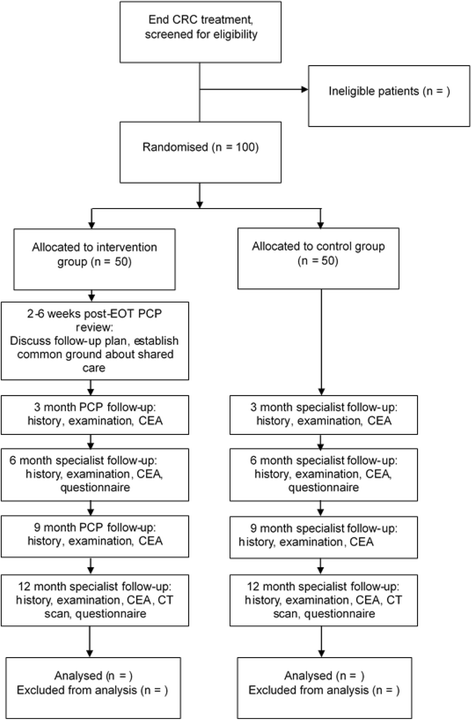
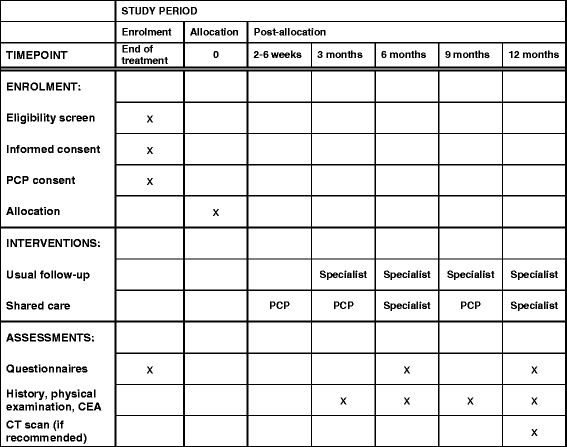
Methods/design: SCORE (Shared care of Colorectal cancer survivors) is a multisite randomised controlled trial designed to optimise and operationalise a shared care model for survivors of CRC, to evaluate the acceptability of the intervention and study processes, and to collect preliminary data regarding the effects of shared care compared with usual care on a range of patient-reported outcomes. The primary outcome is QoL measured using the European Organisation for Research and Treatment of Cancer QLQ-C30 questionnaire. Secondary outcomes are satisfaction with care, unmet needs, continuity of care and health resource use. The shared care model involves replacement of two routine specialist follow-up visits with PCP visits, as well as the provision of a tailored survivorship care plan and a survivorship booklet and DVD for CRC survivors. All consenting patients will be randomised 1:1 to either shared care or usual care and will complete questionnaires at three time points over a 12-month period (baseline and at 6 and 12 months). Health care resource use data will also be collected and used to evaluate costs.
Discussion: The evaluation and implementation of models of care that are responsive to the holistic needs of cancer survivors while reducing the burden on acute care settings is an international priority. Shared care between specialists and PCPs has the potential to enhance patient care and outcomes for CRC survivors while offering improvements in health care resource efficiency. If the findings of the present study show that the shared care intervention is acceptable and feasible for CRC survivors, the intervention may be readily expanded to other groups of cancer survivors.
Trial registration: Australian New Zealand Clinical Trials Registry, ACTRN12617000004369p . Registered on 3 January 2017; protocol version 4 approved 24 February 2017.
Keywords: Colorectal cancer; Follow-up; Models of care; Primary care; Protocol; Shared care; Survivorship.
Conflict of interest statement
Ethics approval and consent to participate
Ethics approval was received 15 December 2016 from the Peter MacCallum Cancer Centre Human Research Ethics Committee (HREC/16/PMCC/89). Amendments to the study will be submitted to ethic committees and updated on the trial registry. Participants have provided or will provide written informed consent to participate in the trial.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Kopp I, Bauhofer A, Koller M. Understanding quality of life in patients with colorectal cancer: comparison of data from a randomised controlled trial, a population based cohort study and the norm reference population. Inflamm Res. 2004;53(Suppl 2):S130–5. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

