The long non-coding RNA ROCR contributes to SOX9 expression and chondrogenic differentiation of human mesenchymal stem cells
- PMID: 29084806
- PMCID: PMC5769619
- DOI: 10.1242/dev.152504
The long non-coding RNA ROCR contributes to SOX9 expression and chondrogenic differentiation of human mesenchymal stem cells
Abstract
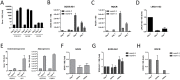
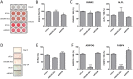
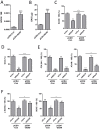
Long non-coding RNAs (lncRNAs) are expressed in a highly tissue-specific manner and function in various aspects of cell biology, often as key regulators of gene expression. In this study, we established a role for lncRNAs in chondrocyte differentiation. Using RNA sequencing we identified a human articular chondrocyte repertoire of lncRNAs from normal hip cartilage donated by neck of femur fracture patients. Of particular interest are lncRNAs upstream of the master chondrocyte transcription factor SOX9 locus. SOX9 is an HMG-box transcription factor that plays an essential role in chondrocyte development by directing the expression of chondrocyte-specific genes. Two of these lncRNAs are upregulated during chondrogenic differentiation of mesenchymal stem cells (MSCs). Depletion of one of these lncRNAs, LOC102723505, which we termed ROCR (regulator of chondrogenesis RNA), by RNA interference disrupted MSC chondrogenesis, concomitant with reduced cartilage-specific gene expression and incomplete matrix component production, indicating an important role in chondrocyte biology. Specifically, SOX9 induction was significantly ablated in the absence of ROCR, and overexpression of SOX9 rescued the differentiation of MSCs into chondrocytes. Our work sheds further light on chondrocyte-specific SOX9 expression and highlights a novel method of chondrocyte gene regulation involving a lncRNA.
Keywords: Cartilage; Chondrogenesis; Differentiation; Epigenetics; LncRNA; MSC; SOX9.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures



References
-
- Akiyama H., Chaboissier M. C., Martin J. F., Schedl A. and de Crombrugghe B. (2002). The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 16, 2813-2828. 10.1101/gad.1017802 - DOI - PMC - PubMed
-
- Barter M. J., Tselepi M., Gómez R., Woods S., Hui W., Smith G. R., Shanley D. P., Clark I. M. and Young D. A. (2015). Genome-wide microRNA and gene analysis of mesenchymal stem cell chondrogenesis identifies an essential role and multiple targets for miR-140-5p. Stem Cells 33, 3266-3280. 10.1002/stem.2093 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

