Angiotensin II-derived constrained peptides with antiplasmodial activity and suppressed vasoconstriction
- PMID: 29085013
- PMCID: PMC5662717
- DOI: 10.1038/s41598-017-14642-z
Angiotensin II-derived constrained peptides with antiplasmodial activity and suppressed vasoconstriction
Abstract
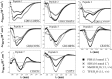
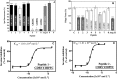
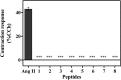
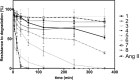
Angiotensin II (Ang II) is a natural mammalian hormone that has been described to exhibit antiplasmodial activity therefore constituting a promising alternative for the treatment of malaria. Despite its promise, the development of Ang II as an antimalarial is limited by its potent induction of vasoconstriction and its rapid degradation within minutes. Here, we used peptide design to perform targeted chemical modifications to Ang II to generate conformationally restricted (disulfide-crosslinked) peptide derivatives with suppressed vasoconstrictor activity and increased stability. Designed constrained peptides were synthesized chemically and then tested for antiplasmodial activity. Two lead constrained peptides were identified (i.e., peptides 1 and 2), each composed of 10 amino acid residues. These peptides exhibited very promising activity in both our Plasmodium gallinaceum (>80%) and Plasmodium falciparum (>40%) models, an activity that was equivalent to that of Ang II, and led to complete suppression of vasoconstriction. In addition, peptide 5 exhibited selective activity towards the pre-erythrocytic stage (98% of activity against P. gallinaceum), thus suggesting that it may be possible to design peptides that target specific stages of the malaria life cycle. The Ang II derived stable scaffolds presented here may provide the basis for development of a new generation of peptide-based drugs for the treatment of malaria.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- World Health Organization. Global Healthy Observatory (GHO) data. http://www.who.int/gho/malaria/en/ (2015).
-
- UNICEF. https://data.unicef.org/topic/child-health/malaria/# (2015).
-
- Silva, A. F. et al. Antiplasmodial activity study of angiotensin II via Ala scan analogs. J Pept Sci (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

