Biomechanical, ultrastructural, and electrophysiological characterization of the non-human primate experimental glaucoma model
- PMID: 29085025
- PMCID: PMC5662689
- DOI: 10.1038/s41598-017-14720-2
Biomechanical, ultrastructural, and electrophysiological characterization of the non-human primate experimental glaucoma model
Abstract
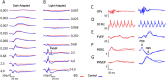
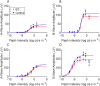
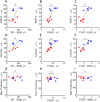
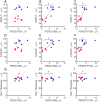
Laser-induced experimental glaucoma (ExGl) in non-human primates (NHPs) is a common animal model for ocular drug development. While many features of human hypertensive glaucoma are replicated in this model, structural and functional changes in the unlasered portions of trabecular meshwork (TM) of laser-treated primate eyes are understudied. We studied NHPs with ExGl of several years duration. As expected, ExGl eyes exhibited selective reductions of the retinal nerve fiber layer that correlate with electrophysiologic measures documenting a link between morphologic and elctrophysiologic endpoints. Softening of unlasered TM in ExGl eyes compared to untreated controls was observed. The degree of TM softening was consistent, regardless of pre-mortem clinical findings including severity of IOP elevation, retinal nerve fiber layer thinning, or electrodiagnostic findings. Importantly, this softening is contrary to TM stiffening reported in glaucomatous human eyes. Furthermore, microscopic analysis of unlasered TM from eyes with ExGl demonstrated TM thinning with collapse of Schlemm's canal; and proteomic analysis confirmed downregulation of metabolic and structural proteins. These data demonstrate unexpected and compensatory changes involving the TM in the NHP model of ExGl. The data suggest that compensatory mechanisms exist in normal animals and respond to elevated IOP through softening of the meshwork to increase outflow.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

