Dynamic biofilm architecture confers individual and collective mechanisms of viral protection
- PMID: 29085075
- PMCID: PMC5739289
- DOI: 10.1038/s41564-017-0050-1
Dynamic biofilm architecture confers individual and collective mechanisms of viral protection
Abstract
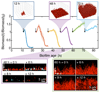
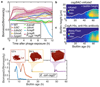
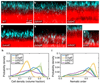
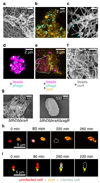
In nature, bacteria primarily live in surface-attached, multicellular communities, termed biofilms 1-6 . In medical settings, biofilms cause devastating damage during chronic and acute infections; indeed, bacteria are often viewed as agents of human disease 7 . However, bacteria themselves suffer from diseases, most notably in the form of viral pathogens termed bacteriophages 8-12 , which are the most abundant replicating entities on Earth. Phage-biofilm encounters are undoubtedly common in the environment, but the mechanisms that determine the outcome of these encounters are unknown. Using Escherichia coli biofilms and the lytic phage T7 as models, we discovered that an amyloid fibre network of CsgA (curli polymer) protects biofilms against phage attack via two separate mechanisms. First, collective cell protection results from inhibition of phage transport into the biofilm, which we demonstrate in vivo and in vitro. Second, CsgA fibres protect cells individually by coating their surface and binding phage particles, thereby preventing their attachment to the cell exterior. These insights into biofilm-phage interactions have broad-ranging implications for the design of phage applications in biotechnology, phage therapy and the evolutionary dynamics of phages with their bacterial hosts.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Comment in
-
Phaged and confused by biofilm matrix.Nat Microbiol. 2018 Jan;3(1):2-3. doi: 10.1038/s41564-017-0078-2. Nat Microbiol. 2018. PMID: 29255281 No abstract available.
References
-
- Flemming H-C, et al. Biofilms: an emergent form of bacterial life. Nat Rev Microbiol. 2016;14:563–75. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

