Evaluation of Visual Field and Imaging Outcomes for Glaucoma Clinical Trials (An American Ophthalomological Society Thesis)
- PMID: 29085257
- PMCID: PMC5652981
Evaluation of Visual Field and Imaging Outcomes for Glaucoma Clinical Trials (An American Ophthalomological Society Thesis)
Abstract
Purpose: To evaluate the ability of various visual field (VF) analysis methods to discriminate treatment groups in glaucoma clinical trials and establish the value of time-domain optical coherence tomography (TD OCT) imaging as an additional outcome.
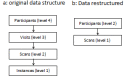
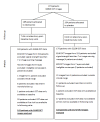
Methods: VFs and retinal nerve fibre layer thickness (RNFLT) measurements (acquired by TD OCT) from 373 glaucoma patients in the UK Glaucoma Treatment Study (UKGTS) at up to 11 scheduled visits over a 2 year interval formed the cohort to assess the sensitivity of progression analysis methods. Specificity was assessed in 78 glaucoma patients with up to 11 repeated VF and OCT RNFLT measurements over a 3 month interval. Growth curve models assessed the difference in VF and RNFLT rate of change between treatment groups. Incident progression was identified by 3 VF-based methods: Guided Progression Analysis (GPA), 'ANSWERS' and 'PoPLR', and one based on VFs and RNFLT: 'sANSWERS'. Sensitivity, specificity and discrimination between treatment groups were evaluated.
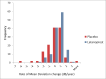
Results: The rate of VF change was significantly faster in the placebo, compared to active treatment, group (-0.29 vs +0.03 dB/year, P<.001); the rate of RNFLT change was not different (-1.7 vs -1.1 dB/year, P=.14). After 18 months and at 95% specificity, the sensitivity of ANSWERS and PoPLR was similar (35%); sANSWERS achieved a sensitivity of 70%. GPA, ANSWERS and PoPLR discriminated treatment groups with similar statistical significance; sANSWERS did not discriminate treatment groups.
Conclusions: Although the VF progression-detection method including VF and RNFLT measurements is more sensitive, it does not improve discrimination between treatment arms.
Figures











References
-
- Weinreb RN, Kaufman PL. The glaucoma research community and FDA look to the future: a report from the NEI/FDA CDER Glaucoma Clinical Trial Design and Endpoints Symposium. Invest Ophthalmol Vis Sci. 2009;50(4):1497–1505. - PubMed
-
- Henson DB, Chaudry S, Artes PH, Faragher EB, Ansons A. Response variability in the visual field: comparison of optic neuritis, glaucoma, ocular hypertension, and normal eyes. Invest Ophthalmol Vis Sci. 2000;41(2):417–421. - PubMed
-
- Artes PH, Iwase A, Ohno Y, Kitazawa Y, Chauhan BC. Properties of perimetric threshold estimates from Full Threshold, SITA Standard, and SITA Fast strategies. Invest Ophthalmol Vis Sci. 2002;43(8):2654–2659. - PubMed
-
- Russell RA, Crabb DP, Malik R, Garway-Heath DF. The relationship between variability and sensitivity in large-scale longitudinal visual field data. Invest Ophthalmol Vis Sci. 2012;53(10):5985–5990. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
