Safeguards of Neurotransmission: Endocytic Adaptors as Regulators of Synaptic Vesicle Composition and Function
- PMID: 29085282
- PMCID: PMC5649181
- DOI: 10.3389/fncel.2017.00320
Safeguards of Neurotransmission: Endocytic Adaptors as Regulators of Synaptic Vesicle Composition and Function
Abstract
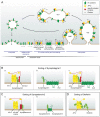
Communication between neurons relies on neurotransmitters which are released from synaptic vesicles (SVs) upon Ca2+ stimuli. To efficiently load neurotransmitters, sense the rise in intracellular Ca2+ and fuse with the presynaptic membrane, SVs need to be equipped with a stringently controlled set of transmembrane proteins. In fact, changes in SV protein composition quickly compromise neurotransmission and most prominently give rise to epileptic seizures. During exocytosis SVs fully collapse into the presynaptic membrane and consequently have to be replenished to sustain neurotransmission. Therefore, surface-stranded SV proteins have to be efficiently retrieved post-fusion to be used for the generation of a new set of fully functional SVs, a process in which dedicated endocytic sorting adaptors play a crucial role. The question of how the precise reformation of SVs is achieved is intimately linked to how SV membranes are retrieved. For a long time both processes were believed to be two sides of the same coin since Clathrin-mediated endocytosis (CME), the proposed predominant SV recycling mode, will jointly retrieve SV membranes and proteins. However, with the recent proposal of Clathrin-independent SV recycling pathways SV membrane retrieval and SV reformation turn into separable events. This review highlights the progress made in unraveling the molecular mechanisms mediating the high-fidelity retrieval of SV proteins and discusses how the gathered knowledge about SV protein recycling fits in with the new notions of SV membrane endocytosis.
Keywords: AP180; presynapse; recycling; release site clearance; sorting; stonin2.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

