Group 3 Innate Lymphoid Cells: Communications Hubs of the Intestinal Immune System
- PMID: 29085366
- PMCID: PMC5649144
- DOI: 10.3389/fimmu.2017.01298
Group 3 Innate Lymphoid Cells: Communications Hubs of the Intestinal Immune System
Abstract
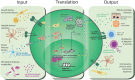
The maintenance of mammalian health requires the generation of appropriate immune responses against a broad range of environmental and microbial challenges, which are continually encountered at barrier tissue sites including the skin, lung, and gastrointestinal tract. Dysregulated barrier immune responses result in inflammation, both locally and systemically in peripheral organs. Group 3 innate lymphoid cells (ILC3) are constitutively present at barrier sites and appear to be highly specialized in their ability to sense a range of environmental and host-derived signals. Under homeostatic conditions, ILC3 respond to local cues to maintain tissue homeostasis and restrict inflammatory responses. In contrast, perturbations in the tissue microenvironment resulting from disease, infection, or tissue damage can drive dysregulated pro-inflammatory ILC3 responses and contribute to immunopathology. The tone of the ILC3 response is dictated by a balance of "exogenous" signals, such as dietary metabolites and commensal microbes, and "endogenous" host-derived signals from stromal cells, immune cells, and the nervous system. ILC3 must therefore have the capacity to simultaneously integrate a wide array of complex and dynamic inputs in order to regulate barrier function and tissue health. In this review, we discuss the concept of ILC3 as a "communications hub" in the intestinal tract and associated lymphoid tissues and address the variety of signals, derived from multiple biological systems, which are interpreted by ILC3 to modulate the release of downstream effector molecules and regulate cell-cell crosstalk. Successful integration of environmental cues by ILC3 and downstream propagation to the broader immune system is required to maintain a tolerogenic and anti-inflammatory tone and reinforce barrier function, whereas dysregulation of ILC3 responses can contribute to the onset or progression of clinically relevant chronic inflammatory diseases.
Keywords: group 3 innate lymphoid cell; inflammatory bowel disease; innate lymphoid cells; interleukin-22; intestinal inflammation.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

