Sugar or Fat?-Metabolic Requirements for Immunity to Viral Infections
- PMID: 29085369
- PMCID: PMC5649203
- DOI: 10.3389/fimmu.2017.01311
Sugar or Fat?-Metabolic Requirements for Immunity to Viral Infections
Abstract
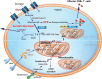
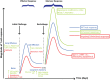
The realization that an intricate link exists between the metabolic state of immune cells and the nature of the elicited immune responses has brought a dramatic evolution to the field of immunology. We will focus on how metabolic reprogramming through the use of glycolysis and fatty-acid oxidation (sugar or fat) regulates the capacity of immune cells to mount robust and effective immune responses. We will also discuss how fine-tuning sugar and fat metabolism may be exploited as a novel immunotherapeutic strategy to fight viral infections or improve vaccine efficacy.
Keywords: HIV; T cells; glycolysis; immunometabolism; mTOR; viral infection.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

