A compact microfluidic chip with integrated impedance biosensor for protein preconcentration and detection
- PMID: 29085524
- PMCID: PMC5653376
- DOI: 10.1063/1.4996118
A compact microfluidic chip with integrated impedance biosensor for protein preconcentration and detection
Abstract
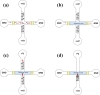
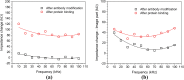
In this study, a low-cost, compact biochip is designed and fabricated for protein detection. Nanofractures formed by self-assembled gold nanoparticles at junction gaps are applied for ion enrichment and depletion to create a trapping zone when electroosmotic flow occurs in microchannels. An impedance measurement module is implemented based on the lock-in amplifier technique to measure the impedance change during antibody growth on the gold electrodes which is caused by trapped proteins in the detection region. The impedance measurement results confirm the presence of trapped proteins. Distinguishable impedance profiles, measured at frequencies in the range of 10-100 kHz, for the detection area taken before and after the presence of proteins validate the performance of the proposed system.
Figures





References
LinkOut - more resources
Full Text Sources
Other Literature Sources
