Passive Stretch Induces Structural and Functional Maturation of Engineered Heart Muscle as Predicted by Computational Modeling
- PMID: 29086457
- PMCID: PMC5785460
- DOI: 10.1002/stem.2732
Passive Stretch Induces Structural and Functional Maturation of Engineered Heart Muscle as Predicted by Computational Modeling
Abstract
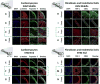
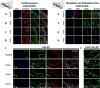
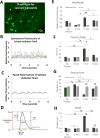
The ability to differentiate human pluripotent stem cells (hPSCs) into cardiomyocytes (CMs) makes them an attractive source for repairing injured myocardium, disease modeling, and drug testing. Although current differentiation protocols yield hPSC-CMs to >90% efficiency, hPSC-CMs exhibit immature characteristics. With the goal of overcoming this limitation, we tested the effects of varying passive stretch on engineered heart muscle (EHM) structural and functional maturation, guided by computational modeling. Human embryonic stem cells (hESCs, H7 line) or human induced pluripotent stem cells (IMR-90 line) were differentiated to hPSC-derived cardiomyocytes (hPSC-CMs) in vitro using a small molecule based protocol. hPSC-CMs were characterized by troponin+ flow cytometry as well as electrophysiological measurements. Afterwards, 1.2 × 106 hPSC-CMs were mixed with 0.4 × 106 human fibroblasts (IMR-90 line) (3:1 ratio) and type-I collagen. The blend was cast into custom-made 12-mm long polydimethylsiloxane reservoirs to vary nominal passive stretch of EHMs to 5, 7, or 9 mm. EHM characteristics were monitored for up to 50 days, with EHMs having a passive stretch of 7 mm giving the most consistent formation. Based on our initial macroscopic observations of EHM formation, we created a computational model that predicts the stress distribution throughout EHMs, which is a function of cellular composition, cellular ratio, and geometry. Based on this predictive modeling, we show cell alignment by immunohistochemistry and coordinated calcium waves by calcium imaging. Furthermore, coordinated calcium waves and mechanical contractions were apparent throughout entire EHMs. The stiffness and active forces of hPSC-derived EHMs are comparable with rat neonatal cardiomyocyte-derived EHMs. Three-dimensional EHMs display increased expression of mature cardiomyocyte genes including sarcomeric protein troponin-T, calcium and potassium ion channels, β-adrenergic receptors, and t-tubule protein caveolin-3. Passive stretch affects the structural and functional maturation of EHMs. Based on our predictive computational modeling, we show how to optimize cell alignment and calcium dynamics within EHMs. These findings provide a basis for the rational design of EHMs, which enables future scale-up productions for clinical use in cardiovascular tissue engineering. Stem Cells 2018;36:265-277.
Keywords: Bioengineering; Calcium handling; Cardiac; Cardiomyocyte; Computational modeling; Engineered heart muscle; Heart; Pluripotent stem cells; Tissue engineering; Tissue regeneration.
© 2017 AlphaMed Press.
Conflict of interest statement
J.R. is an employee of Genentech. Evangeline Tzatzalos discloses an advisory role with Wilson Sonsini Goodrich and is an employee of Rosati. D.B. is a patent holder for RegenCor (collagen patch for cardiac regeneration). All other authors indicated no potential conflicts of interest.
Figures




References
-
- Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics– 2010 update: A report from the American Heart Association. Circulation. 2010;121:e46–e215. - PubMed
-
- Healy KE, McDevitt TC, Murphy WL, et al. Engineering the emergence of stem cell therapeutics. Sci Transl Med. 2013;5:207ed217. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

