Endothelial cell-derived GABA signaling modulates neuronal migration and postnatal behavior
- PMID: 29086765
- PMCID: PMC5799810
- DOI: 10.1038/cr.2017.135
Endothelial cell-derived GABA signaling modulates neuronal migration and postnatal behavior
Abstract
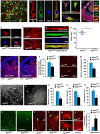
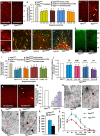
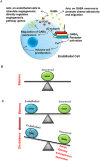
The cerebral cortex is essential for integration and processing of information that is required for most behaviors. The exquisitely precise laminar organization of the cerebral cortex arises during embryonic development when neurons migrate successively from ventricular zones to coalesce into specific cortical layers. While radial glia act as guide rails for projection neuron migration, pre-formed vascular networks provide support and guidance cues for GABAergic interneuron migration. This study provides novel conceptual and mechanistic insights into this paradigm of vascular-neuronal interactions, revealing new mechanisms of GABA and its receptor-mediated signaling via embryonic forebrain endothelial cells. With the use of two new endothelial cell specific conditional mouse models of the GABA pathway (Gabrb3ΔTie2-Cre and VgatΔTie2-Cre), we show that partial or complete loss of GABA release from endothelial cells during embryogenesis results in vascular defects and impairs long-distance migration and positioning of cortical interneurons. The downstream effects of perturbed endothelial cell-derived GABA signaling are critical, leading to lasting changes to cortical circuits and persistent behavioral deficits. Furthermore, we illustrate new mechanisms of activation of GABA signaling in forebrain endothelial cells that promotes their migration, angiogenesis and acquisition of blood-brain barrier properties. Our findings uncover and elucidate a novel endothelial GABA signaling pathway in the CNS that is distinct from the classical neuronal GABA signaling pathway and shed new light on the etiology and pathophysiology of neuropsychiatric diseases, such as autism spectrum disorders, epilepsy, anxiety, depression and schizophrenia.
Figures






References
-
- Treiman DM. GABAergic mechanisms in epilepsy. Epilepsia 2001; 42 Suppl 3:8–12. - PubMed
-
- Marin O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci 2012; 13:107–120. - PubMed
-
- Levitt P, Eagleson KL, Powell EM. Regulation of neocortical interneuron development and the implications for neurodevelopmental disorders. Trends Neurosci 2004; 27:400–406. - PubMed
-
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci 2005; 6:312–324. - PubMed
-
- Holmes GL, Ben-Ari Y. The neurobiology and consequences of epilepsy in the developing brain. Pediatr Res 2001; 49:320–325. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

