DsbA-L prevents obesity-induced inflammation and insulin resistance by suppressing the mtDNA release-activated cGAS-cGAMP-STING pathway
- PMID: 29087318
- PMCID: PMC5699051
- DOI: 10.1073/pnas.1708744114
DsbA-L prevents obesity-induced inflammation and insulin resistance by suppressing the mtDNA release-activated cGAS-cGAMP-STING pathway
Abstract
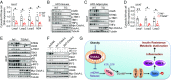
Chronic inflammation in adipose tissue plays a key role in obesity-induced insulin resistance. However, the mechanisms underlying obesity-induced inflammation remain elusive. Here we show that obesity promotes mtDNA release into the cytosol, where it triggers inflammatory responses by activating the DNA-sensing cGAS-cGAMP-STING pathway. Fat-specific knockout of disulfide-bond A oxidoreductase-like protein (DsbA-L), a chaperone-like protein originally identified in the mitochondrial matrix, impaired mitochondrial function and promoted mtDNA release, leading to activation of the cGAS-cGAMP-STING pathway and inflammatory responses. Conversely, fat-specific overexpression of DsbA-L protected mice against high-fat diet-induced activation of the cGAS-cGAMP-STING pathway and inflammation. Taken together, we identify DsbA-L as a key molecule that maintains mitochondrial integrity. DsbA-L deficiency promotes inflammation and insulin resistance by activating the cGAS-cGAMP-STING pathway. Our study also reveals that, in addition to its well-characterized roles in innate immune surveillance, the cGAS-cGAMP-STING pathway plays an important role in mediating obesity-induced metabolic dysfunction.
Keywords: DsbA-L; cGAS; inflammation; insulin resistance; obesity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
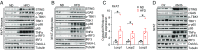


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

