Surgical Rates for Crohn's Disease are Decreasing: A Population-Based Time Trend Analysis and Validation Study
- PMID: 29087396
- PMCID: PMC5729339
- DOI: 10.1038/ajg.2017.394
Surgical Rates for Crohn's Disease are Decreasing: A Population-Based Time Trend Analysis and Validation Study
Erratum in
-
Corrigendum: Surgical Rates for Crohn's Disease Are Decreasing: A Population-Based Time Trend Analysis and Validation Study.Am J Gastroenterol. 2018 Feb;113(2):310. doi: 10.1038/ajg.2017.468. Epub 2017 Dec 19. Am J Gastroenterol. 2018. PMID: 29257148
Abstract
Objectives: Temporal changes for intestinal resections for Crohn's disease (CD) are controversial. We validated administrative database codes for CD diagnosis and surgery in hospitalized patients and then evaluated temporal trends in CD surgical resection rates.
Methods: First, we validated International Classification of Disease (ICD)-10-CM coding for CD diagnosis in hospitalized patients and Canadian Classification of Health Intervention coding for surgical resections. Second, we used these validated codes to conduct population-based surveillance between fiscal years 2002 and 2010 to identify adult CD patients undergoing intestinal resection (n=981). Annual surgical rate was calculated by dividing incident surgeries by estimated CD prevalence. Time trend analysis was performed and annual percent change (APC) with 95% confidence intervals (CI) in surgical resection rates were calculated using a generalized linear model assuming a Poisson distribution.
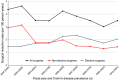
Results: In the validation cohort, 101/104 (97.1%) patients undergoing surgery and 191/200 (95.5%) patients admitted without surgery were confirmed to have CD on chart review. Among the 116 administrative database codes for surgical resection, 97.4% were confirmed intestinal resections on chart review. From 2002 to 2010, the overall CD surgical resection rate was 3.8 resections per 100 person-years. During the study period, rate of surgery decreased by 3.5% per year (95% CI: -1.1%, -5.8%), driven by decreasing emergent operations (-10.1% per year (95% CI: -13.4%, -6.7%)) whereas elective surgeries increased by 3.7% per year (95% CI: 0.1%, 7.3%).
Conclusions: Overall surgical resection rates in CD are decreasing, but a paradigm shift has occurred whereby elective operations are now more commonly performed than emergent surgeries.
Conflict of interest statement
Figures
References
-
- Hazlewood GS, Rezaie A, Borman M et al. Comparative effectiveness of immunosuppressants and biologics for inducing and maintaining remission in Crohn's disease: a network meta-analysis. Gastroenterology 2015;148:344–354. - PubMed
-
- Singh S, Al-Darmaki A, Frolkis AD et al. Postoperative mortality among patients with inflammatory bowel diseases: a systematic review and meta-analysis of population-based studies. Gastroenterology 2015;149:928–937. - PubMed
-
- Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology 2016;152:313–321.e2. - PubMed
-
- Fichera A, Michelassi F. Surgical treatment of Crohn's disease. J Gastrointest Surg 2007;11:791–803. - PubMed
-
- Frolkis AD, Dykeman J, Negron ME et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology 2013;145:996–1006. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

