Neutralization of Human Cytomegalovirus Entry into Fibroblasts and Epithelial Cells
- PMID: 29088098
- PMCID: PMC5748606
- DOI: 10.3390/vaccines5040039
Neutralization of Human Cytomegalovirus Entry into Fibroblasts and Epithelial Cells
Abstract
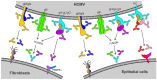
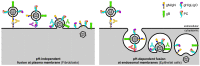
Human cytomegalovirus (HCMV) is a leading cause of permanent birth defects, highlighting the need to develop an HCMV vaccine candidate. However, HCMV vaccine development is complicated by the varying capacity of neutralizing antibodies (NAb) to interfere in vitro with the HCMV entry routes mediating infection of fibroblast (FB) and epithelial cells (EC). While HCMV infection of FB and EC requires glycoprotein complexes composed of gB and gH/gL/gO, EC infection depends additionally on the envelope pentamer complex (PC) composed of gH, gL, UL128, UL130 and UL131A. Unlike NAb to gB or gH epitopes that can interfere with both FB and EC infection, NAb targeting predominantly conformational epitopes of the UL128/130/131A subunits are unable to prevent FB entry, though they are highly potent in blocking EC infection. Despite the selective requirement of the PC for EC entry, the PC is exceptionally immunogenic as vaccine antigen to stimulate both EC- and FB-specific NAb responses due to its capacity to elicit NAb that target epitopes of the UL128/130/131A subunits and gH. These findings suggest that the PC could be sufficient in a subunit vaccine formulation to induce robust FB- and EC-specific NAb responses. In this short review, we discuss NAb responses induced through natural infection and vaccination that interfere in vitro with HCMV infection of FB and EC.
Keywords: cytomegalovirus; epithelial cells; fibroblasts; glycoprotein complex; neutralizing antibody; pentamer; vaccine.
Conflict of interest statement
All authors receive research support from Helocyte Inc. Don J. Diamond, Felix Wussow and Flavia Chiuppesi receive royalty payments from Helocyte Inc. Don J. Diamond chairs the Scientific Advisory Board and has an equity interest in Helocyte Inc.
Figures

References
-
- Britt W. Manifestations of human cytomegalovirus infection: Proposed mechanisms of acute and chronic disease. Curr. Top. Microbiol. Immunol. 2008;325:417–470. - PubMed
-
- Stratton K.R., Durch J.S., Lawrence R.S. Vaccines for the 21st Century: A Tool for Decisionmaking. The National Academies Press; Washington, DC, USA: 2000. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

