TMEM132: an ancient architecture of cohesin and immunoglobulin domains define a new family of neural adhesion molecules
- PMID: 29088312
- PMCID: PMC6030884
- DOI: 10.1093/bioinformatics/btx689
TMEM132: an ancient architecture of cohesin and immunoglobulin domains define a new family of neural adhesion molecules
Abstract
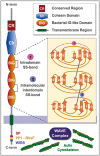
Summary: The molecular functions of TMEM132 genes remain poorly understood and under-investigated despite their mutations associated with non-syndromic hearing loss, panic disorder and cancer. Here we show the full domain architecture of human TMEM132 family proteins solved using in-depth sequence and structural analysis. We reveal them to be five previously unappreciated cell adhesion molecules whose domain architecture has an early holozoan origin prior to the emergence of choanoflagellates and metazoa. The extra-cellular portions of TMEM132 proteins contain five conserved domains including three tandem immunoglobulin domains, and a cohesin domain homologue, the first such domain found in animals. These findings strongly predict a cellular adhesion function for TMEM132 family, connecting the extracellular medium with the intracellular actin cytoskeleton.
Contact: luis.sanchez-pulido@igmm.ed.ac.uk.
Supplementary information: Supplementary data are available at Bioinformatics online.
© The Author(s) 2017. Published by Oxford University Press.
Figures
References
-
- Abedin M., King N. (2008) The premetazoan ancestry of cadherins. Science, 319, 946–948. - PubMed
-
- Artzi L. et al. (2017) Cellulosomes: bacterial nanomachines for dismantling plant polysaccharides. Nat. Rev. Microbiol., 15, 83–95. - PubMed
-
- Bork P. et al. (1994) The immunoglobulin fold. Structural classification, sequence patterns and common core. J. Mol. Biol., 242, 309–320. - PubMed
-
- Brieher W.M., Yap A.S. (2013) Cadherin junctions and their cytoskeleton(s). Curr. Opin. Cell Biol., 25, 39–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

