Kctd13 deletion reduces synaptic transmission via increased RhoA
- PMID: 29088697
- PMCID: PMC5787033
- DOI: 10.1038/nature24470
Kctd13 deletion reduces synaptic transmission via increased RhoA
Abstract
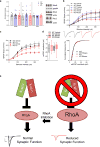
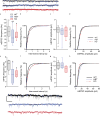
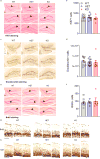
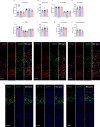
Copy-number variants of chromosome 16 region 16p11.2 are linked to neuropsychiatric disorders and are among the most prevalent in autism spectrum disorders. Of many 16p11.2 genes, Kctd13 has been implicated as a major driver of neurodevelopmental phenotypes. The function of KCTD13 in the mammalian brain, however, remains unknown. Here we delete the Kctd13 gene in mice and demonstrate reduced synaptic transmission. Reduced synaptic transmission correlates with increased levels of Ras homolog gene family, member A (RhoA), a KCTD13/CUL3 ubiquitin ligase substrate, and is reversed by RhoA inhibition, suggesting increased RhoA as an important mechanism. In contrast to a previous knockdown study, deletion of Kctd13 or kctd13 does not increase brain size or neurogenesis in mice or zebrafish, respectively. These findings implicate Kctd13 in the regulation of neuronal function relevant to neuropsychiatric disorders and clarify the role of Kctd13 in neurogenesis and brain size. Our data also reveal a potential role for RhoA as a therapeutic target in disorders associated with KCTD13 deletion.
Conflict of interest statement
The authors declare competing financial interests: details are available in the online version of the paper. Readers are welcome to comment on the online version of the paper.
Figures








References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

