Sulforaphane reduces YAP/∆Np63α signaling to reduce cancer stem cell survival and tumor formation
- PMID: 29088716
- PMCID: PMC5650271
- DOI: 10.18632/oncotarget.20562
Sulforaphane reduces YAP/∆Np63α signaling to reduce cancer stem cell survival and tumor formation
Abstract
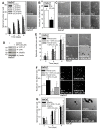
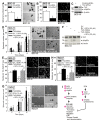
Epidermal squamous cell carcinoma (SCC) is among the most common cancers. SCC can be treated by surgical excision, but recurrence of therapy-resistant disease is a major problem. We recently showed that YAP1, the Hippo signaling transcription adaptor protein, and ∆Np63α, a key epidermal stem cell survival protein, form a complex to drive epidermal cancer stem cell survival. In the present study, we demonstrate that YAP1 and ∆Np63α are important sulforaphane cancer prevention targets. We show that sulforaphane treatment increases YAP1 phosphorylation and proteolytic degradation. The loss of YAP1 is associated with a reduction in ∆Np63α level and a reduction in ECS cell survival, spheroid formation, invasion and migration. Loss of YAP1 and ∆Np63α is mediated by the proteasome and can be inhibited by lactacystin treatment. YAP1 or ∆Np63α knockdown replicates the responses to sulforaphane, and restoration of YAP1 or ∆Np63α antagonizes sulforaphane action. Sulforaphane suppresses ECS cell tumor formation and this is associated with reduced levels of YAP1 and ∆Np63α. These studies suggest that YAP1 and ∆Np63α may be important sulforaphane cancer preventive targets in epidermal squamous cell carcinoma.
Keywords: TAZ; YAP; hippo signaling; sulforaphane; ∆Np63α.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflict of interest
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

