Neuropilin-1 is a glial cell line-derived neurotrophic factor receptor in glioblastoma
- PMID: 29088765
- PMCID: PMC5650320
- DOI: 10.18632/oncotarget.18630
Neuropilin-1 is a glial cell line-derived neurotrophic factor receptor in glioblastoma
Abstract
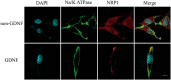
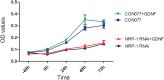
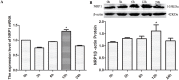
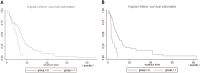
The aim of this study was to identify the receptor for glial cell line-derived neurotrophic factor (GDNF) in glioblastoma multiforme (GBM). After GST pull-down assays, membrane proteins purified from C6 rat glioma cells were subjected to liquid chromatography-tandem mass spectrometry (LC-MS/MS). The differentially expressed proteins were annotated using Gene Ontology, and neuropilin-1 (NRP1) was identified as the putative GDNF receptor in glioma. NRP1 was more highly expressed in human GBM brains and C6 rat glioma cells than in normal human brains or primary rat astrocytes. Immunofluorescence staining showed that NRP1 was recruited to the membrane by GDNF, and NRP1 co-immunoprecipitated with GDNF. Using the NRP1 and GDNF protein structures to assess molecular docking in the ZDOCK server and visualization with the PyMOL Molecular Graphics System revealed 8 H-bonds and stable positive and negative electrostatic interactions between NRP1 and GDNF. RNAi knockdown of NRP1 reduced proliferation of C6 glioma cells when stimulated with GDNF. NRP1 was an independent risk factor for both survival and recurrence in GBM patients. High NRP1 mRNA expression correlated with shorter OS and DFS (OS: χ2=4.6720, P=0.0307; DFS: χ2=11.013, P=0.0009). NRP1 is thus a GDNF receptor in glioma cells and a potential therapeutic target.
Keywords: cell proliferation; glial cell line-derived neurotrophic factor; glioblastoma; membrane receptor; neuropilin-1.
Figures






References
-
- Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, Chin L, DePinho RA, Cavenee WK. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–710. - PubMed
-
- Wiesenhofer B, Stockhammer G, Kostron H, Maier H, Hinterhuber H, Humpel C. Glial cell line-derived neurotrophic factor (GDNF) and its receptor (GFR-alpha 1) are strongly expressed in human gliomas. Acta Neuropathol. 2000;99:131–37. - PubMed
-
- Wiesenhofer B, Weis C, Humpel C. Glial cell line-derived neurotrophic factor (GDNF) is a proliferation factor for rat C6 glioma cells: evidence from antisense experiments. Antisense Nucleic Acid Drug Dev. 2000;10:311–21. - PubMed
-
- Lodish HB, Zipursky SL, Matsudaira P, Baltimore D, Darnell J. Molecular Cell Biology. New York, USA: W.H. Freeman; 2000.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

