The prevention of fragility fractures in patients with non-metastatic prostate cancer: a position statement by the international osteoporosis foundation
- PMID: 29088899
- PMCID: PMC5650454
- DOI: 10.18632/oncotarget.17980
The prevention of fragility fractures in patients with non-metastatic prostate cancer: a position statement by the international osteoporosis foundation
Abstract
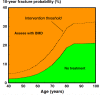
Androgen deprivation therapy is commonly employed for the treatment of non-metastatic prostate cancer as primary or adjuvant treatment. The skeleton is greatly compromised in men with prostate cancer during androgen deprivation therapy because of the lack of androgens and estrogens, which are trophic factors for bone. Men receiving androgen deprivation therapy sustain variable degrees of bone loss with an increased risk of fragility fractures. Several bone antiresorptive agents have been tested in randomized controlled trials in these patients. Oral bisphosphonates, such as alendronate and risedronate, and intravenous bisphosphonates, such as pamidronate and zoledronic acid, have been shown to increase bone density and decrease the risk of fractures in men receiving androgen deprivation therapy. Denosumab, a fully monoclonal antibody that inhibits osteoclastic-mediated bone resorption, is also effective in increasing bone mineral density and reducing fracture rates in these patients. The assessment of fracture risk, T-score and/or the evaluation of prevalent fragility fractures are mandatory for the selection of patients who will benefit from antiresorptive therapy. In the future, new agents modulating bone turnover and skeletal muscle metabolism will be available for testing in these subjects.
Keywords: ADT; FRAX; androgen deprivation therapy; osteoporosis; zoledronic acid.
Conflict of interest statement
CONFLICTS OF INTEREST All authors declare no conflict of interest related to the subject of this paper. More general disclosures: L.C. lecture fees: Abiogen Pharma. B.F. advisory board, consulting fees: Abiogen, Amgen, Bayer, Italfarmaco; lecture fees: Abiogen Pharma, Amgen, Lilly. K.J.A. reports grants from Amgen, grants from Lilly, non-financial support from Medimaps, grants from Unigene, non-financial support from Asahi, grants from Radius Health. Outside the submitted work, he is the architect of FRAX but has no financial interest. R.J.Y.: consulting fees or paid advisory boards: Servier, Novartis, Negma, Lilly, Wyeth, Amgen, GlaxoSmithKline, Roche, Merck, Nycomed, NPS, Theramex, UCB; lecture fees when speaking at the invitation of a commercial sponsor: Merck Sharp and Dohme, Lilly, Rottapharm, IBSA, Genevrier, Novartis, Servier, Roche, GlaxoSmithKline, Teijin, Teva, Ebewee Pharma, Zodiac, Analis, Theramex, Nycomed, Novo-Nordisk, Nolver; grant Support from Industry: Bristol Myers Squibb, Merck Sharp & Dohme, Rottapharm, Teva, Lilly, Novartis, Roche, GlaxoSmithKline, Amgen, Servier. B.M.L. consulting fees and grants from Alexion, Abiogen, Amgen, Bruno Farmaceutici, Eli Lilly, MSD, NPS, Shire, SPA and Servier.
Figures
References
-
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–1403. - PubMed
-
- Arnold M, Karim-Kos HE, Coebergh JW, Byrnes G, Antilla A, Ferlay J, Renehan AG, Forman D, Soerjomataram I. Recent trends in incidence of five common cancers in 26 European countries since 1988: Analysis of the European Cancer Observatory. Eur J Cancer. 2015;51:1164–1187. - PubMed
-
- Filson CP, Marks LS, Litwin MS. Expectant management for men with early stage prostate cancer. CA Cancer J Clin. 2015;65:265–282. - PubMed
-
- Attard G, Parker C, Eeles RA, Schröder F, Tomlins SA, Tannock I, Drake CG, de Bono JS. Prostate cancer. Lancet. 2016;387:70–82. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

