A Randomized, Double-Blind, Placebo-Controlled Trial of Naproxen With or Without Orphenadrine or Methocarbamol for Acute Low Back Pain
- PMID: 29089169
- PMCID: PMC5820149
- DOI: 10.1016/j.annemergmed.2017.09.031
A Randomized, Double-Blind, Placebo-Controlled Trial of Naproxen With or Without Orphenadrine or Methocarbamol for Acute Low Back Pain
Abstract
Study objective: In US emergency departments (EDs), patients with low back pain are often treated with nonsteroidal anti-inflammatory drugs and muscle relaxants. We compare functional outcomes among patients randomized to a 1-week course of naproxen+placebo versus naproxen+orphenadrine or naproxen+methocarbamol.
Methods: This was a randomized, double-blind, comparative effectiveness trial conducted in 2 urban EDs. Patients presenting with acute, nontraumatic, nonradicular low back pain were enrolled. The primary outcome was improvement on the Roland-Morris Disability Questionnaire (RMDQ) between ED discharge and 1 week later. All patients were given 14 tablets of naproxen 500 mg, to be used twice a day, as needed for low back pain. Additionally, patients were randomized to receive a 1-week supply of orphenadrine 100 mg, to be used twice a day as needed, methocarbamol 750 mg, to be used as 1 or 2 tablets 3 times per day as needed, or placebo. All patients received a standardized 10-minute low back pain educational session before discharge.
Results: Two hundred forty patients were randomized. Baseline demographic characteristics were comparable. The mean RMDQ score of patients randomized to naproxen+placebo improved by 10.9 points (95% confidence interval [CI] 8.9 to 12.9). The mean RMDQ score of patients randomized to naproxen+orphenadrine improved by 9.4 points (95% CI 7.4 to 11.5). The mean RMDQ score of patients randomized to naproxen+methocarbamol improved by 8.1 points (95% CI 6.1 to 10.1). None of the between-group differences surpassed our threshold for clinical significance. Adverse events were reported by 17% (95% CI 10% to 28%) of placebo patients, 9% (95% CI 4% to 19%) of orphenadrine patients, and 19% (95% CI 11% to 29%) of methocarbamol patients.
Conclusion: Among ED patients with acute, nontraumatic, nonradicular low back pain, combining naproxen with either orphenadrine or methocarbamol did not improve functional outcomes compared with naproxen+placebo.
Trial registration: ClinicalTrials.gov NCT02665286.
Copyright © 2017 American College of Emergency Physicians. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
We have no conflicts of interest to report.
Figures
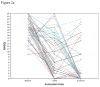


References
-
- Friedman BW, O’Mahony S, Mulvey L, Davitt M, Choi H, Xia S, et al. One-week and 3-month outcomes after an emergency department visit for undifferentiated musculoskeletal low back pain. Annals of emergency medicine. 2012;59(2):128–33. e3. - PubMed
-
- Friedman BW, Dym AA, Davitt M, Holden L, Solorzano C, Esses D, et al. Naproxen With Cyclobenzaprine, Oxycodone/Acetaminophen, or Placebo for Treating Acute Low Back Pain: A Randomized Clinical Trial. JAMA. 2015;314(15):1572–80. - PubMed
-
- Roelofs PD, Deyo RA, Koes BW, Scholten RJ, van Tulder MW. Nonsteroidal anti-inflammatory drugs for low back pain: an updated Cochrane review. Spine. 2008;33(16):1766–74. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

