An Investigation of the Single and Combined Phthalate Metabolite Effects on Human Chorionic Gonadotropin Expression in Placental Cells
- PMID: 29089286
- PMCID: PMC5933420
- DOI: 10.1289/EHP1539
An Investigation of the Single and Combined Phthalate Metabolite Effects on Human Chorionic Gonadotropin Expression in Placental Cells
Abstract
Background: Observational studies have reported associations between maternal phthalate levels and adverse outcomes at birth and in the health of the child. Effects on placental function have been suggested as a biologic basis for these findings.
Objective: We evaluated the effects of phthalates on placental function in vitro by measuring relevant candidate genes and proteins.
Materials and methods: Human trophoblast progenitor cells were isolated at 7-14 wk of pregnancy (two female and three male concepti), and villous cytotrophoblast cells (vCTBs) were isolated at 15-20 wk (three female and four male concepti). Cells were cultured in vitro with four phthalate metabolites and their combination at concentrations based on levels found previously in the urine of pregnant women: mono-n-butyl (MnBP, 200 nM), monobenzyl (MBzP, 3μM), mono-2-ethylhexyl (MEHP, 700 nM), and monoethyl (MEP, 1.5μM) phthalates. mRNA levels of CGA, CGB, PPARG, CYP19A1, CYP11A1, PTGS2, EREG, and the intracellular β subunit of human chorionic gonadotropin (hCGβ) and peroxisome proliferator activated receptor γ (PPARγ) were measured in the cellular extracts, and protein levels for four forms of secreted hCG were measured in the conditioned media.
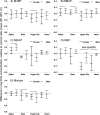
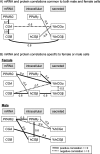
Results: Previously reported associations between maternal phthalates and placental gene expression were reproduced experimentally: MnBP with CGA, MBzP with CYP11A1, and MEHP with PTGS2. CGB and hCGβ were up-regulated by MBzP. In some cases, there were marked, even opposite, differences in response by sex of the cells. There was evidence of agonism in female cells and antagonism in male cells of PPARγ by simultaneous exposure to multiple phthalates.
Conclusions: Concentrations of MnBP, MBzP and MEHP similar to those found in the urine of pregnant women consistently altered hCG and PPARγ expression in primary placental cells. These findings provide evidence for the molecular basis by which phthalates may alter placental function, and they provide a preliminary mechanistic hypothesis for opposite responses by sex. https://doi.org/10.1289/EHP1539.
Figures



References
-
- Adibi JJ, Hauser R, Williams PL, Whyatt RM, Calafat AM, Nelson H, et al. 2009. Maternal urinary metabolites of di-(2-ethylhexyl) phthalate in relation to the timing of labor in a US multicenter pregnancy cohort study. Am J Epidemiol 169(8):1015–1024, PMID: 19251754, 10.1093/aje/kwp001. - DOI - PMC - PubMed
-
- Adibi JJ, Whyatt RM, Hauser R, Bhat HK, Davis BJ, Calafat AM, et al. 2010. Transcriptional biomarkers of steroidogenesis and trophoblast differentiation in the placenta in relation to prenatal phthalate exposure. Environ Health Perspect 118(2):291–296, PMID: 20123604, 10.1289/ehp.0900788. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
