Klebsiella pneumoniae Carbapenemase-2 (KPC-2), Substitutions at Ambler Position Asp179, and Resistance to Ceftazidime-Avibactam: Unique Antibiotic-Resistant Phenotypes Emerge from β-Lactamase Protein Engineering
- PMID: 29089425
- PMCID: PMC5666153
- DOI: 10.1128/mBio.00528-17
Klebsiella pneumoniae Carbapenemase-2 (KPC-2), Substitutions at Ambler Position Asp179, and Resistance to Ceftazidime-Avibactam: Unique Antibiotic-Resistant Phenotypes Emerge from β-Lactamase Protein Engineering
Abstract
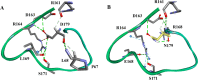
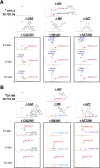
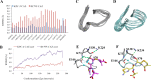
The emergence of Klebsiella pneumoniae carbapenemases (KPCs), β-lactamases that inactivate "last-line" antibiotics such as imipenem, represents a major challenge to contemporary antibiotic therapies. The combination of ceftazidime (CAZ) and avibactam (AVI), a potent β-lactamase inhibitor, represents an attempt to overcome this formidable threat and to restore the efficacy of the antibiotic against Gram-negative bacteria bearing KPCs. CAZ-AVI-resistant clinical strains expressing KPC variants with substitutions in the Ω-loop are emerging. We engineered 19 KPC-2 variants bearing targeted mutations at amino acid residue Ambler position 179 in Escherichia coli and identified a unique antibiotic resistance phenotype. We focus particularly on the CAZ-AVI resistance of the clinically relevant Asp179Asn variant. Although this variant demonstrated less hydrolytic activity, we demonstrated that there was a prolonged period during which an acyl-enzyme intermediate was present. Using mass spectrometry and transient kinetic analysis, we demonstrated that Asp179Asn "traps" β-lactams, preferentially binding β-lactams longer than AVI owing to a decreased rate of deacylation. Molecular dynamics simulations predict that (i) the Asp179Asn variant confers more flexibility to the Ω-loop and expands the active site significantly; (ii) the catalytic nucleophile, S70, is shifted more than 1.5 Å and rotated more than 90°, altering the hydrogen bond networks; and (iii) E166 is displaced by 2 Å when complexed with ceftazidime. These analyses explain the increased hydrolytic profile of KPC-2 and suggest that the Asp179Asn substitution results in an alternative complex mechanism leading to CAZ-AVI resistance. The future design of novel β-lactams and β-lactamase inhibitors must consider the mechanistic basis of resistance of this and other threatening carbapenemases.IMPORTANCE Antibiotic resistance is emerging at unprecedented rates and threatens to reach crisis levels. One key mechanism of resistance is the breakdown of β-lactam antibiotics by β-lactamase enzymes. KPC-2 is a β-lactamase that inactivates carbapenems and β-lactamase inhibitors (e.g., clavulanate) and is prevalent around the world, including in the United States. Resistance to the new antibiotic ceftazidime-avibactam, which was designed to overcome KPC resistance, had already emerged within a year. Using protein engineering, we uncovered a mechanism by which resistance to this new drug emerges, which could arm scientists with the ability to forestall such resistance to future drugs.
Keywords: KPC-2; avibactam; beta-lactam; beta-lactamase; carbapenemase; ceftazidime.
Copyright © 2017 Barnes et al.
Figures


References
-
- General Assembly of the United Nations. 21 September 2016. High-level meeting on antimicrobial resistance. UN Headquarters, New York, NY: http://www.un.org/pga/71/2016/09/21/press-release-hl-meeting-on-antimicr....
-
- Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, Cornaglia G, Garau J, Gniadkowski M, Hayden MK, Kumarasamy K, Livermore DM, Maya JJ, Nordmann P, Patel JB, Paterson DL, Pitout J, Villegas MV, Wang H, Woodford N, Quinn JP. 2013. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis 13:785–796. doi:10.1016/S1473-3099(13)70190-7. - DOI - PMC - PubMed
-
- Winkler ML, Rodkey EA, Taracila MA, Drawz SM, Bethel CR, Papp-Wallace KM, Smith KM, Xu Y, Dwulit-Smith JR, Romagnoli C, Caselli E, Prati F, van den Akker F, Bonomo RA. 2013. Design and exploration of novel boronic acid inhibitors reveals important interactions with a clavulanic acid-resistant sulfhydryl-variable (SHV) β-lactamase. J Med Chem 56:1084–1097. doi:10.1021/jm301490d. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
