In vivo near-infrared imaging of ErbB2 expressing breast tumors with dual-axes confocal endomicroscopy using a targeted peptide
- PMID: 29089571
- PMCID: PMC5663926
- DOI: 10.1038/s41598-017-13735-z
In vivo near-infrared imaging of ErbB2 expressing breast tumors with dual-axes confocal endomicroscopy using a targeted peptide
Abstract
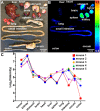
ErbB2 expression in early breast cancer can predict tumor aggressiveness and clinical outcomes in large patient populations. Accurate assessment with physical biopsy and conventional pathology can be limited by tumor heterogeneity. We aim to demonstrate real-time optical sectioning using a near-infrared labeled ErbB2 peptide that generates tumor-specific contrast in human xenograft breast tumors in vivo. We used IRDye800CW as the fluorophore, validated performance characteristics for specific peptide binding to cells in vitro, and investigated peak peptide uptake in tumors using photoacoustic tomography. We performed real-time optical imaging using a handheld dual-axes confocal fluorescence endomicroscope that collects light off-axis to reduce tissue scattering for greater imaging depths. Optical sections in either the vertical or horizontal plane were collected with sub-cellular resolution. Also, we found significantly greater peptide binding to pre-clinical xenograft breast cancer in vivo and to human specimens of invasive ductal carcinoma that express ErbB2 ex vivo. We used a scrambled peptide for control. Peptide biodistribution showed high tumor uptake by comparison with other organs to support safety. This novel integrated imaging strategy is promising for visualizing ErbB2 expression in breast tumors and serve as an adjunct during surgery to improve diagnostic accuracy, identify tumor margins, and stage early cancers.
Conflict of interest statement
Authors are inventors on patents filed by the University of Michigan on the peptide (BPJ and TDW) and the endomicroscope (GL, HL, XD, and TDW) presented in this study. The other authors declare that they have no competing interests.
Figures





Similar articles
-
Application of a CYP1B1-Targeted NIR Probe for Breast Cancer Diagnosis, Surgical Navigation, and CYP1B1-Associated Chemotherapy Resistance Monitoring.Mol Pharm. 2025 Mar 3;22(3):1507-1517. doi: 10.1021/acs.molpharmaceut.4c01223. Epub 2025 Jan 30. Mol Pharm. 2025. PMID: 39885683
-
Dual-labeled trastuzumab-based imaging agent for the detection of human epidermal growth factor receptor 2 overexpression in breast cancer.J Nucl Med. 2007 Sep;48(9):1501-10. doi: 10.2967/jnumed.107.042234. J Nucl Med. 2007. PMID: 17785729
-
Evaluation of an 111In-radiolabeled peptide as a targeting and imaging agent for ErbB-2 receptor expressing breast carcinomas.Clin Cancer Res. 2007 Oct 15;13(20):6070-9. doi: 10.1158/1078-0432.CCR-07-0160. Clin Cancer Res. 2007. PMID: 17947470
-
Impact of molecular subtypes classification concordance between preoperative core needle biopsy and surgical specimen on early breast cancer management: Single-institution experience and review of published literature.Eur J Surg Oncol. 2017 Apr;43(4):642-648. doi: 10.1016/j.ejso.2016.10.025. Epub 2016 Nov 17. Eur J Surg Oncol. 2017. PMID: 27889196 Review.
-
Optical Imaging of the Breast: Basic Principles and Clinical Applications.AJR Am J Roentgenol. 2017 Jul;209(1):230-238. doi: 10.2214/AJR.16.17220. Epub 2017 Apr 5. AJR Am J Roentgenol. 2017. PMID: 28379746 Review.
Cited by
-
Development of ErbB2-Targeting Liposomes for Enhancing Drug Delivery to ErbB2-Positive Breast Cancer.Pharmaceutics. 2020 Jun 24;12(6):585. doi: 10.3390/pharmaceutics12060585. Pharmaceutics. 2020. PMID: 32599712 Free PMC article.
-
A Conformationally Restricted Aza-BODIPY Platform for Stimulus-Responsive Probes with Enhanced Photoacoustic Properties.J Am Chem Soc. 2019 Nov 6;141(44):17601-17609. doi: 10.1021/jacs.9b06694. Epub 2019 Oct 29. J Am Chem Soc. 2019. PMID: 31660741 Free PMC article.
-
Dual-axis confocal microscopy for point-of-care pathology.IEEE J Sel Top Quantum Electron. 2019 Jan-Feb;25(1):7100910. doi: 10.1109/JSTQE.2018.2854572. Epub 2018 Jul 25. IEEE J Sel Top Quantum Electron. 2019. PMID: 30872909 Free PMC article.
-
Miniature side-view dual axes confocal endomicroscope for repetitive in vivo imaging.Biomed Opt Express. 2023 Jul 26;14(8):4277-4295. doi: 10.1364/BOE.494210. eCollection 2023 Aug 1. Biomed Opt Express. 2023. PMID: 37799693 Free PMC article.
-
A Facile and Reproducible Synthesis of Near-Infrared Fluorescent Conjugates with Small Targeting Molecules for Microbial Infection Imaging.ACS Omega. 2020 Aug 26;5(35):22071-22080. doi: 10.1021/acsomega.0c02094. eCollection 2020 Sep 8. ACS Omega. 2020. PMID: 32923765 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

