ACVR2B/Fc counteracts chemotherapy-induced loss of muscle and bone mass
- PMID: 29089584
- PMCID: PMC5665981
- DOI: 10.1038/s41598-017-15040-1
ACVR2B/Fc counteracts chemotherapy-induced loss of muscle and bone mass
Abstract
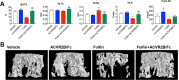
Chemotherapy promotes the development of cachexia, a debilitating condition characterized by muscle and fat loss. ACVR2B/Fc, an inhibitor of the Activin Receptor 2B signaling, has been shown to preserve muscle mass and prolong survival in tumor hosts, and to increase bone mass in models of osteogenesis imperfecta and muscular dystrophy. We compared the effects of ACVR2B/Fc on muscle and bone mass in mice exposed to Folfiri. In addition to impairing muscle mass and function, Folfiri had severe negative effects on bone, as shown by reduced trabecular bone volume fraction (BV/TV), thickness (Tb.Th), number (Tb.N), connectivity density (Conn.Dn), and by increased separation (Tb.Sp) in trabecular bone of the femur and vertebra. ACVR2B/Fc prevented the loss of muscle mass and strength, and the loss of trabecular bone in femurs and vertebrae following Folfiri administration. Neither Folfiri nor ACVR2B/Fc had effects on femoral cortical bone, as shown by unchanged cortical bone volume fraction (Ct.BV/TV), thickness (Ct.Th) and porosity. Our results suggest that Folfiri is responsible for concomitant muscle and bone degeneration, and that ACVR2B/Fc prevents these derangements. Future studies are required to determine if the same protective effects are observed in combination with other anticancer regimens or in the presence of cancer.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures








References
-
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA: a cancer journal for clinicians. 2017;67:7–30. - PubMed
-
- Costelli P, Baccino FM. Mechanisms of skeletal muscle depletion in wasting syndromes: role of ATP-ubiquitin-dependent proteolysis. Current opinion in clinical nutrition and metabolic care. 2003;6:407–412. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

