Incentivising innovation in antibiotic drug discovery and development: progress, challenges and next steps
- PMID: 29089600
- PMCID: PMC5746591
- DOI: 10.1038/ja.2017.124
Incentivising innovation in antibiotic drug discovery and development: progress, challenges and next steps
Abstract
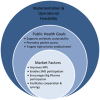
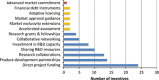
Political momentum and funding for combatting antimicrobial resistance (AMR) continues to build. Numerous major international and national initiatives aimed at financially incentivising the research and development (R&D) of antibiotics have been implemented. However, it remains unclear how to effectively strengthen the current set of incentive programmes to further accelerate antibiotic innovation. Based on a literature review and expert input, this study first identifies and assesses the major international, European Union, US and UK antibiotic R&D funding programmes. These programmes are then evaluated across market and public health criteria necessary for comprehensively improving the antibiotic market. The current set of incentive programmes are an important initial step to improving the economic feasibility of antibiotic development. However, there appears to be a lack of global coordination across all initiatives, which risks duplicating efforts, leaving funding gaps in the value chain and overlooking important AMR goals. This study finds that incentive programmes are overly committed to early-stage push funding of basic science and preclinical research, while there is limited late-stage push funding of clinical development. Moreover, there are almost no pull incentives to facilitate transition of antibiotic products from early clinical phases to commercialisation, focus developer concentration on the highest priority antibiotics and attract large pharmaceutical companies to invest in the market. Finally, it seems that antibiotic sustainability and patient access requirements are poorly integrated into the array of incentive mechanisms.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- The Review on Antimicrobial Resistance. Antimicrobial resistance: tackling a crisis for the health and wealth of nations, London UK, 1–20, https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Ta... (2014).
-
- Rex, J. H. et al. A comprehensive regulatory framework to address the unmet need for new antibacterial treatments. Lancet Infect Dis. 13, 269–275 (2013). - PubMed
-
- Outterson, K., Powers, J. H., Daniel, G. W. & McClellan, M. B. Repairing the broken market for antibiotic innovation. Health Aff. (Millwood) 34, 277–285 (2015). - PubMed
-
- The Boston Consulting Group. Breaking through the wall: enhancing research and development of antibiotics in science and industry, Berlin DE, 1–84, https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/5_Publikat... (2017).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

