miR-1296-5p decreases ERBB2 expression to inhibit the cell proliferation in ERBB2-positive breast cancer
- PMID: 29089858
- PMCID: PMC5655974
- DOI: 10.1186/s12935-017-0466-y
miR-1296-5p decreases ERBB2 expression to inhibit the cell proliferation in ERBB2-positive breast cancer
Abstract
Background: The tumor suppressive role of miR-1296 is observed in triple negative breast cancer (TNBC). However, the effect of miR-1296-5p in ERBB2-positive breast cancers remains obscure.
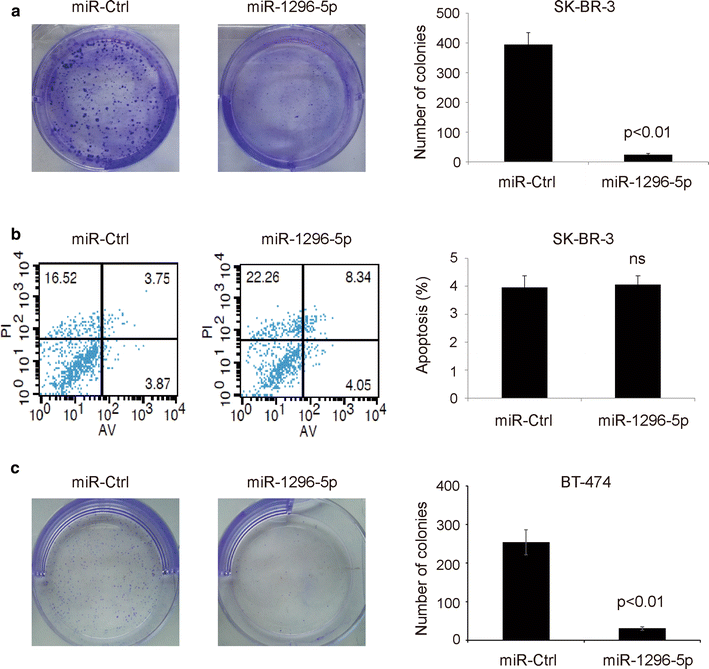
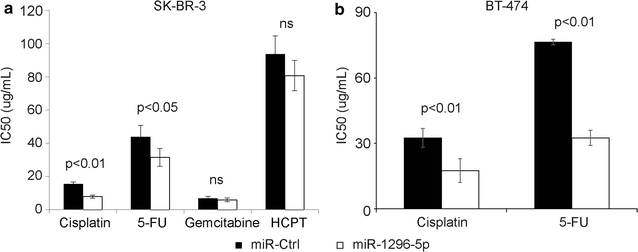
Methods: Whether ERBB2 was the target gene of the miR-1296-5p was predicted by online software, and determined by dual-luciferase activity assay. miR-1296-5p expression levels were determined in breast cancer samples (114 breast cancer tissues and 30 adjacent normal tissues) by using qRT-PCR. The effect of miR-1296-5p and inhibition of ERBB2/mTORC1 signaling on the downstream target was assessed by Western blot. SK-BR-3 and BT-474 breast cancer cell line was transfected with miR-1296-5p mimic after which cell proliferation and apoptosis were determined by the clonogenic assay and the flow cytometry system, respectively. In addition, the chemotherapeutic drug sensitivity of SK-BR-3 and BT-474 cells transfected with miR-1296-5p mimic were determined by MTT assay.
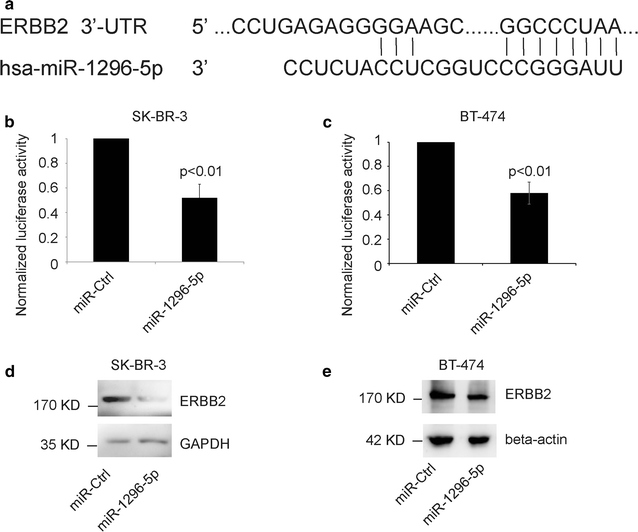
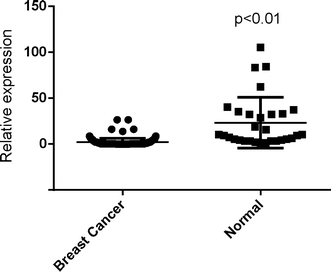
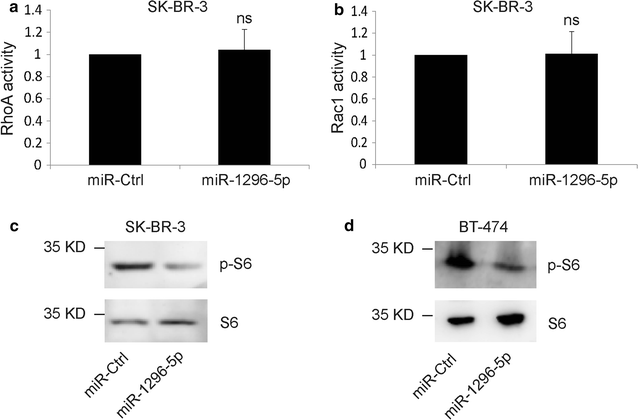
Results: The luciferase assay carrying ERBB2 3'-untranslated region-based reporters expressed in SK-BR-3 and BT-474 cells suggested that ERBB2 was the target gene of miR-1296-5p. MiR-1296-5p was significantly decreased in breast cancer tissues compared to adjacent normal tissues. Moreover, it was declined in ERBB2-positive breast cancer samples compared with that in ERBB2-negative breast cancer tissues. Overexpressed miR-1296-5p reduced its target protein level and mTORC1/S6 activation, inhibited the proliferation of ERBB2-positive breast cancer cells and sensitized these cells to cisplatin and 5-fluorouracil-induced apoptosis.
Conclusions: Our findings suggest that miR-1296-5p is involved in the regulation of proliferation in breast cancer cells via targeting ERBB2/mTORC1 signaling pathway.
Keywords: Breast cancer; ERBB2; Proliferation; mTORC1; miR-1296-5p.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

