Ion Channel Trafficking: Control of Ion Channel Density as a Target for Arrhythmias?
- PMID: 29089904
- PMCID: PMC5650974
- DOI: 10.3389/fphys.2017.00808
Ion Channel Trafficking: Control of Ion Channel Density as a Target for Arrhythmias?
Abstract
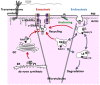
The shape of the cardiac action potential (AP) is determined by the contributions of numerous ion channels. Any dysfunction in the proper function or expression of these ion channels can result in a change in effective refractory period (ERP) and lead to arrhythmia. The processes underlying the correct targeting of ion channels to the plasma membrane are complex, and have not been fully characterized in cardiac myocytes. Emerging evidence highlights ion channel trafficking as a potential causative factor in certain acquired and inherited arrhythmias, and therapies which target trafficking as opposed to pore block are starting to receive attention. In this review we present the current evidence for the mechanisms which underlie precise control of cardiac ion channel trafficking and targeting.
Keywords: accessory proteins; arrhythmias; cardiac; potassium channels; sodium channel; trafficking.
Figures
References
-
- Balse E., El-Haou S., Dillanian G., Dauphin A., Eldstrom J., Fedida D., et al. (2009). Cholesterol modulates the recruitment of Kv1.5 channels from Rab11-associated recycling endosome in native atrial myocytes. Proc. Natl. Acad. Sci. U.S.A. 106, 14681–14686. 10.1073/pnas.0902809106 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

