Production of Bacterial Cellulose by Gluconacetobacter hansenii Using Corn Steep Liquor As Nutrient Sources
- PMID: 29089941
- PMCID: PMC5651021
- DOI: 10.3389/fmicb.2017.02027
Production of Bacterial Cellulose by Gluconacetobacter hansenii Using Corn Steep Liquor As Nutrient Sources
Abstract
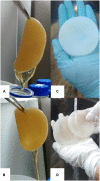
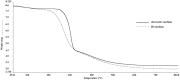
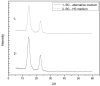
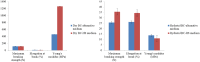
Cellulose is mainly produced by plants, although many bacteria, especially those belonging to the genus Gluconacetobacter, produce a very peculiar form of cellulose with mechanical and structural properties that can be exploited in numerous applications. However, the production cost of bacterial cellulose (BC) is very high to the use of expensive culture media, poor yields, downstream processing, and operating costs. Thus, the purpose of this work was to evaluate the use of industrial residues as nutrients for the production of BC by Gluconacetobacter hansenii UCP1619. BC pellicles were synthesized using the Hestrin-Schramm (HS) medium and alternative media formulated with different carbon (sugarcane molasses and acetylated glucose) and nitrogen sources [yeast extract, peptone, and corn steep liquor (CSL)]. A jeans laundry was also tested. None of the tested sources (beside CSL) worked as carbon and nutrient substitute. The alternative medium formulated with 1.5% glucose and 2.5% CSL led to the highest yield in terms of dry and hydrated mass. The BC mass produced in the alternative culture medium corresponded to 73% of that achieved with the HS culture medium. The BC pellicles demonstrated a high concentration of microfibrils and nanofibrils forming a homogenous, compact, and three-dimensional structure. The biopolymer produced in the alternative medium had greater thermal stability, as degradation began at 240°C, while degradation of the biopolymer produced in the HS medium began at 195°C. Both biopolymers exhibited high crystallinity. The mechanical tensile test revealed the maximum breaking strength and the elongation of the break of hydrated and dry pellicles. The dry BC film supported up to 48 MPa of the breaking strength and exhibited greater than 96.98% stiffness in comparison with the hydrated film. The dry film supported up to 48 MPa of the breaking strength and exhibited greater than 96.98% stiffness in comparison with the hydrated film. The values obtained for the Young's modulus in the mechanical tests in the hydrated samples indicated low values for the variable rigidity. The presence of water in the interior and between the nanofibers of the hydrated BC only favored the results for the elasticity, which was 56.37% higher when compared to the dry biomaterial.
Keywords: Gluconacetobacter hansenii; bacterial cellulose; carbon source; nitrogen source; waste.
Figures


References
-
- Ashjaran A., Yazdanshenas M. E., Rashidi A., Khajavi R., Rezaee A. (2013). Overview of bio nanofabric from bacterial cellulose. J. Text. Inst. 104 121–131. 10.1080/00405000.2012.703796 - DOI
-
- Bertazzoli R., Pelegrini R. (2002). Descoloração e degradação de poluentes orgânicos em soluções aquosas do processo fotoeletroquímico. Quím. Nova 25 477–482. 10.1590/S0100-40422002000300022 - DOI
-
- Borzani W., Souza S. J. (1995). Mechanism of the film thickness increasing during the bacterial production of cellulose on non-agitated liquid media. Biotechnol. Lett. 17 1271–1272. 10.1007/BF00128400 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

